Assessment of anti-mutagenic, anti-histopathologic and antioxidant capacities of Egyptian bee pollen and propolis extracts
- PMID: 23677589
- PMCID: PMC3918268
- DOI: 10.1007/s10616-013-9568-0
Assessment of anti-mutagenic, anti-histopathologic and antioxidant capacities of Egyptian bee pollen and propolis extracts
Abstract
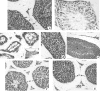
Bee pollen and propolis are popular, traditional health foods. The objective of the current study was to investigate the anti-mutagenic, anti-histopathologic and antioxidant effects among water extracts of Egyptian bee pollen (WEBP) and brown powder of water-soluble derivative propolis (WSDP) on cisplatin (CDDP) induced hepatic, renal, testicular and genotoxicity in male albino mice (Mus muscullus), in addition to their effects on the oxidant/antioxidant status in the tested organs. Hepatic, renal and testicular dysfunctions were evaluated histologically; while genotoxicity and cytotoxicity were evaluated by the bone marrow chromosomal aberration assay and mitotic index, respectively. Moreover, oxidative stress was explored via determination of lipid peroxidation, catalase activity and the concentration of the reduced form of glutathione. The treatment of mice with WEBP and WSDP at doses 140 and 8.4 mg/kg b. wt./day, respectively for 14 days simultaneously with CDDP (2.8 mg/kg b. wt.) resulted in significant protection. The positive control animals taken CDDP alone showed toxic histological and genetical manifestations (at P < 0.05) accompanied with an elevated content of peroxidized lipid and lowered catalase activity and glutathione concentration in the homogenate of liver, kidney and testis tissues (at P < 0.001). These toxic side effects in all tested organs were greatly ablated with a significant reduction in lipid peroxidation level and elevation in catalase activity and glutathione concentration (P < 0.001) when using both WEBP and WSDP. On the basis of the present assays, Bee pollen appears more potent in exerting an ameliorative effect and this effect was more pronounced in testis.
Figures



References
-
- Abdella E, Ahmed R. Suppression of doxorubicin apoptotic, histopathologic, mutagenic and oxidative stress effect in mice bone marrow and tested tissue by aqueous Rosemary leave extract. Egypt J Zool. 2008;51:305–330.
-
- Ahmed R, Abdella E. Modulatory effect of Rosemary leave aqueous extract on doxorubicin-induced histological lesions, apoptosis and oxidative stress in mice. J Egypt Ger Soc Zool. 2009;57C:105–137.
-
- Aly M, Ashour M, El Nahas S, Abo Zeid M. Genotoxicity and cytotoxicity of the anticancer drugs gemcitabine and cisplatin, separately and in comination: in vivo studies. J Biol Sci. 2003;3(11):961–972. doi: 10.3923/jbs.2003.961.972. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

