An optogenetic tool for the activation of endogenous diaphanous-related formins induces thickening of stress fibers without an increase in contractility
- PMID: 23677607
- PMCID: PMC4064713
- DOI: 10.1002/cm.21115
An optogenetic tool for the activation of endogenous diaphanous-related formins induces thickening of stress fibers without an increase in contractility
Abstract
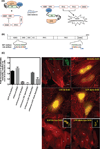
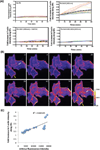
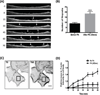
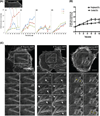
We have developed an optogenetic technique for the activation of diaphanous-related formins. Our approach is based on fusion of the light-oxygen-voltage 2 domain of Avena sativa Phototrophin1 to an isolated Diaphanous Autoregulatory Domain from mDia1. This "caged" diaphanous auto-regulatory domain was inactive in the dark but in the presence of blue light rapidly activated endogenous diaphanous-related formins. Using an F-actin reporter, we observed filopodia and lamellipodia formation as well as a steady increase in F-actin along existing stress fibers, starting within minutes of photo-activation. Interestingly, we did not observe the formation of new stress fibers. Remarkably, a 1.9-fold increase in F-actin was not paralleled by an increase in myosin II along stress fibers and the amount of tension generated by the fibers, as judged by focal adhesion size, appeared unchanged. Our results suggest a decoupling between F-actin accumulation and contractility in stress fibers and demonstrate the utility of photoactivatable diaphanous autoregulatory domain for the study of diaphanous-related formin function in cells.
Keywords: actin polymerization; actomyosin contractility; diaphanous-related formins; light-oxygen-voltage domain; optogenetics; stress fiber.
Copyright © 2013 Wiley Periodicals, Inc.
Figures


References
-
- Alberts AS. Identification of a carboxyl-terminal diaphanous-related formin homology protein autoregulatory domain. J Biol Chem. 2001;276(4):2824–2830. - PubMed
-
- Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275(5304):1308–1311. - PubMed
-
- Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol. 2001;3(5):466–472. - PubMed
-
- Bershadsky AD, Ballestrem C, Carramusa L, Zilberman Y, Gilquin B, Khochbin S, Alexandrova AY, Verkhovsky AB, Shemesh T, Kozlov MM. Assembly and mechanosensory function of focal adhesions: experiments and models. Eur J Cell Biol. 2006;85(3–4):165–173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

