Localized induction of the ATP-binding cassette B19 auxin transporter enhances adventitious root formation in Arabidopsis
- PMID: 23677937
- PMCID: PMC3707546
- DOI: 10.1104/pp.113.217174
Localized induction of the ATP-binding cassette B19 auxin transporter enhances adventitious root formation in Arabidopsis
Abstract
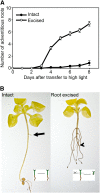
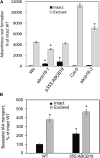
Adventitious roots emerge from aerial plant tissues, and the induction of these roots is essential for clonal propagation of agriculturally important plant species. This process has received extensive study in horticultural species but much less focus in genetically tractable model species. We have explored the role of auxin transport in this process in Arabidopsis (Arabidopsis thaliana) seedlings in which adventitious root initiation was induced by excising roots from low-light-grown hypocotyls. Inhibition of auxin transport from the shoot apex abolishes adventitious root formation under these conditions. Root excision was accompanied by a rapid increase in radioactive indole-3-acetic acid (IAA) transport and its accumulation in the hypocotyl above the point of excision where adventitious roots emerge. Local increases in auxin-responsive gene expression were also observed above the site of excision using three auxin-responsive reporters. These changes in auxin accumulation preceded cell division events, monitored by a cyclin B1 reporter (pCYCB1;1:GUS), and adventitious root initiation. We examined excision-induced adventitious root formation in auxin influx and efflux mutants, including auxin insensitive1, pin-formed1 (pin1), pin2, pin3, and pin7, with the most profound reductions observed in ATP-binding cassette B19 (ABCB19). An ABCB19 overexpression line forms more adventitious roots than the wild type in intact seedlings. Examination of transcriptional and translational fusions between ABCB19 and green fluorescent protein indicates that excision locally induced the accumulation of ABCB19 transcript and protein that is temporally and spatially linked to local IAA accumulation leading to adventitious root formation. These experiments are consistent with localized synthesis of ABCB19 protein after hypocotyl excision leads to enhanced IAA transport and local IAA accumulation driving adventitious root formation.
Figures





References
-
- Barkawi LS, Tam YY, Tillman JA, Pederson B, Calio J, Al-Amier H, Emerick M, Normanly J, Cohen JD. (2008) A high-throughput method for the quantitative analysis of indole-3-acetic acid and other auxins from plant tissue. Anal Biochem 372: 177–188 - PubMed
-
- Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J. (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602 - PubMed
-
- Celenza JL, Jr, Grisafi PL, Fink GR. (1995) A pathway for lateral root formation in Arabidopsis thaliana. Genes Dev 9: 2131–2142 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

