Deregulation of apoptotic factors Bcl-xL and Bax confers apoptotic resistance to myeloid-derived suppressor cells and contributes to their persistence in cancer
- PMID: 23677993
- PMCID: PMC3696683
- DOI: 10.1074/jbc.M112.434530
Deregulation of apoptotic factors Bcl-xL and Bax confers apoptotic resistance to myeloid-derived suppressor cells and contributes to their persistence in cancer
Abstract
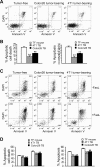
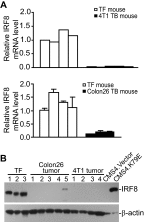
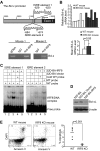
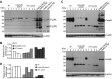
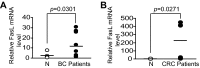
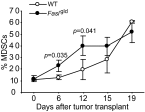
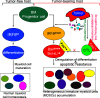
Myeloid-derived suppressor cells (MDSCs) are heterogeneous immature myeloid cells that accumulate in response to tumor progression. Compelling data from mouse models and human cancer patients showed that tumor-induced inflammatory mediators induce MDSC differentiation. However, the mechanisms underlying MDSC persistence is largely unknown. Here, we demonstrated that tumor-induced MDSCs exhibit significantly decreased spontaneous apoptosis as compared with myeloid cells with the same phenotypes from tumor-free mice. Consistent with the decreased apoptosis, cell surface Fas receptor decreased significantly in tumor-induced MDSCs. Screening for changes of key apoptosis mediators downstream the Fas receptor revealed that expression levels of IRF8 and Bax are diminished, whereas expression of Bcl-xL is increased in tumor-induced MDSCs. We further determined that IRF8 binds directly to Bax and Bcl-x promoter in primary myeloid cells in vivo, and IRF8-deficient MDSC-like cells also exhibit increased Bcl-xL and decreased Bax expression. Analysis of CD69 and CD25 levels revealed that cytotoxic T lymphocytes (CTLs) are partially activated in tumor-bearing hosts. Strikingly, FasL but not perforin and granzymes were selectively activated in CTLs in the tumor-bearing host. ABT-737 significantly increased the sensitivity of MDSCs to Fas-mediated apoptosis in vitro. More importantly, ABT-737 therapy increased MDSC spontaneous apoptosis and decreased MDSC accumulation in tumor-bearing mice. Our data thus determined that MDSCs use down-regulation of IRF8 to alter Bax and Bcl-xL expression to deregulate the Fas-mediated apoptosis pathway to evade elimination by host CTLs. Therefore, targeting Bcl-xL is potentially effective in suppression of MDSC persistence in cancer therapy.
Keywords: Apoptosis; Bax; Bcl-2 Family; Fas; Myeloid Cell.
Figures



Similar articles
-
Myeloid-derived suppressor cells express the death receptor Fas and apoptose in response to T cell-expressed FasL.Blood. 2011 May 19;117(20):5381-90. doi: 10.1182/blood-2010-11-321752. Epub 2011 Mar 30. Blood. 2011. PMID: 21450901 Free PMC article.
-
Regulatory T cells and myeloid-derived suppressor cells in the tumor microenvironment undergo Fas-dependent cell death during IL-2/αCD40 therapy.J Immunol. 2014 Jun 15;192(12):5821-9. doi: 10.4049/jimmunol.1400404. Epub 2014 May 7. J Immunol. 2014. PMID: 24808361 Free PMC article.
-
Tumor-induced myeloid-derived suppressor cell subsets exert either inhibitory or stimulatory effects on distinct CD8+ T-cell activation events.Eur J Immunol. 2013 Nov;43(11):2930-42. doi: 10.1002/eji.201343349. Epub 2013 Aug 25. Eur J Immunol. 2013. PMID: 23878002
-
Mechanisms overseeing myeloid-derived suppressor cell production in neoplastic disease.Cancer Immunol Immunother. 2017 Aug;66(8):989-996. doi: 10.1007/s00262-017-1963-5. Epub 2017 Feb 21. Cancer Immunol Immunother. 2017. PMID: 28224211 Free PMC article. Review.
-
Regulating the suppressors: apoptosis and inflammation govern the survival of tumor-induced myeloid-derived suppressor cells (MDSC).Cancer Immunol Immunother. 2012 Aug;61(8):1319-25. doi: 10.1007/s00262-012-1269-6. Epub 2012 May 1. Cancer Immunol Immunother. 2012. PMID: 22546994 Free PMC article. Review.
Cited by
-
Upregulated TRAIL and Reduced DcR2 Mediate Apoptosis of Decidual PMN-MDSC in Unexplained Recurrent Pregnancy Loss.Front Immunol. 2020 Jun 30;11:1345. doi: 10.3389/fimmu.2020.01345. eCollection 2020. Front Immunol. 2020. PMID: 32695113 Free PMC article.
-
Expression profiles and function of IL6 in polymorphonuclear myeloid-derived suppressor cells.Cancer Immunol Immunother. 2020 Nov;69(11):2233-2245. doi: 10.1007/s00262-020-02620-w. Epub 2020 Jun 1. Cancer Immunol Immunother. 2020. PMID: 32488308 Free PMC article.
-
Arsenic trioxide inhibits tumor-induced myeloid-derived suppressor cells and enhances T-cell activity.Oncol Lett. 2017 Apr;13(4):2141-2150. doi: 10.3892/ol.2017.5679. Epub 2017 Feb 6. Oncol Lett. 2017. PMID: 28454374 Free PMC article.
-
Autocrine IL6-Mediated Activation of the STAT3-DNMT Axis Silences the TNFα-RIP1 Necroptosis Pathway to Sustain Survival and Accumulation of Myeloid-Derived Suppressor Cells.Cancer Res. 2020 Aug 1;80(15):3145-3156. doi: 10.1158/0008-5472.CAN-19-3670. Epub 2020 Jun 17. Cancer Res. 2020. PMID: 32554751 Free PMC article.
-
Myeloid-Derived Suppressive Cells Deficient in Liver X Receptor α Protected From Autoimmune Hepatitis.Front Immunol. 2021 Aug 26;12:732102. doi: 10.3389/fimmu.2021.732102. eCollection 2021. Front Immunol. 2021. PMID: 34512667 Free PMC article.
References
-
- Haile L. A., Gamrekelashvili J., Manns M. P., Korangy F., Greten T. F. (2010) CD49d is a new marker for distinct myeloid-derived suppressor cell subpopulations in mice. J. Immunol. 185, 203–210 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

