Negative regulation of the acetyltransferase TIP60-p53 interplay by UHRF1 (ubiquitin-like with PHD and RING finger domains 1)
- PMID: 23677994
- PMCID: PMC3707659
- DOI: 10.1074/jbc.M113.476606
Negative regulation of the acetyltransferase TIP60-p53 interplay by UHRF1 (ubiquitin-like with PHD and RING finger domains 1)
Abstract
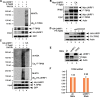
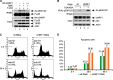
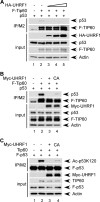
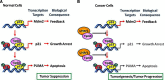
Numerous studies indicate the importance of acetylation in p53-mediated stress responses upon DNA damage. We and others previously showed that TIP60 (Tat-interacting protein of 60 kDa)-mediated acetylation of p53 at K120 is crucial for p53-dependent apoptotic responses. Nevertheless, it remains unclear how TIP60-mediated effects on p53 are dynamically regulated in vivo. Here, we report that UHRF1 (ubiquitin-like with PHD and RING finger domains 1) interacts with TIP60 both in vitro and in vivo and induces degradation-independent ubiquitination of TIP60. Moreover, UHRF1 expression markedly suppresses the ability of TIP60 to acetylate p53. In contrast, RNAi-mediated knockdown of UHRF1 increases the endogenous levels of p53 acetylation at K120 and p53-mediated apoptosis is significantly enhanced in UHRF1-depleted cells. To elucidate the mechanisms of this regulation, we found that the interaction between TIP60 and p53 is severely inhibited in the presence of UHRF1, suggesting that UHRF1 modulates TIP60-mediated functions in both K120 acetylation-dependent and -independent manners. Consistent with this notion, UHRF1 knockdown promotes activation of p21 and PUMA but not MDM2. These findings demonstrate that UHRF1 is a critical negative regulator of TIP60 and suggest that UHRF1-mediated effects on p53 may contribute, at least in part, to its role in tumorigenesis.
Keywords: Acetylation; Apoptosis; Growth Arrest; Oncogene; TIP60; Tumor Suppressor Gene; UHRF1; Ubiquitination; p53.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

