Biochemical features of a catalytic antibody light chain, 22F6, prepared from human lymphocytes
- PMID: 23677996
- PMCID: PMC3707657
- DOI: 10.1074/jbc.M113.454579
Biochemical features of a catalytic antibody light chain, 22F6, prepared from human lymphocytes
Abstract
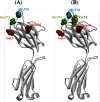
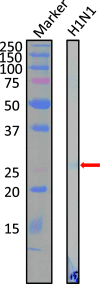
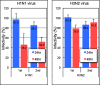
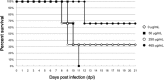
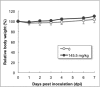
Human antibody light chains belonging to subgroup II of germ line genes were amplified by a seminested PCR technique using B-lymphocytes taken from a human adult infected with influenza virus. Each gene of the human light chains was transferred into the Escherichia coli system. The recovered light chain was highly purified using a two-step purification system. Light chain 22F6 showed interesting catalytic features. The light chain cleaved a peptide bond of synthetic peptidyl-4-methyl-coumaryl-7-amide (MCA) substrates, such as QAR-MCA and EAR-MCA, indicating amidase activity. It also hydrolyzed a phosphodiester bond of both DNA and RNA. From the analysis of amino acid sequences and molecular modeling, the 22F6 light chain possesses two kinds of active sites as amidase and nuclease in close distances. The 22F6 catalytic light chain could suppress the infection of influenza virus type A (H1N1) of Madin-Darby canine kidney cells in an in vitro assay. In addition, the catalytic light chain clearly inhibited the infection of the influenza virus of BALB/c mice via nasal administration in an in vivo assay. In the experiment, the titer in the serum of the mice coinfected with the 22F6 light chain and H1N1 virus became considerably lowered compared with that of 22F6-non-coinfected mice. Note that the catalytic light chain was prepared from human peripheral lymphocyte and plays an important role in preventing infection by influenza virus. Considering the fact that the human light chain did not show any acute toxicity for mice, our procedure developed in this study must be unique and noteworthy for developing new drugs.
Keywords: Catalytic Antibody; DNase; Enzyme Catalysis; Human Light Chain; Influenza Virus; Influenza Virus Type A; Molecular Modeling; RNA Catalysis.
Figures




Similar articles
-
Biochemical features and antiviral activity of a monomeric catalytic antibody light-chain 23D4 against influenza A virus.FASEB J. 2015 Jun;29(6):2347-58. doi: 10.1096/fj.14-264275. Epub 2015 Feb 20. FASEB J. 2015. PMID: 25713031
-
[Preparation and identification of monoclonal antibody against H1N1 influenza virus].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2017 May;33(5):688-694. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2017. PMID: 28502308 Chinese.
-
Potential Role of Nonneutralizing IgA Antibodies in Cross-Protective Immunity against Influenza A Viruses of Multiple Hemagglutinin Subtypes.J Virol. 2020 Jun 1;94(12):e00408-20. doi: 10.1128/JVI.00408-20. Print 2020 Jun 1. J Virol. 2020. PMID: 32269119 Free PMC article.
-
Influenza Neuraminidase Subtype N1: Immunobiological Properties and Functional Assays for Specific Antibody Response.PLoS One. 2016 Apr 7;11(4):e0153183. doi: 10.1371/journal.pone.0153183. eCollection 2016. PLoS One. 2016. PMID: 27054879 Free PMC article.
-
Neutralizing anti-influenza virus monoclonal antibodies: therapeutics and tools for discovery.Int Rev Immunol. 2009;28(1):69-92. doi: 10.1080/08830180802593540. Int Rev Immunol. 2009. PMID: 19241254 Review.
Cited by
-
A new algorithm to convert a normal antibody into the corresponding catalytic antibody.Sci Adv. 2020 Mar 25;6(13):eaay6441. doi: 10.1126/sciadv.aay6441. eCollection 2020 Mar. Sci Adv. 2020. PMID: 32232151 Free PMC article.
-
A new catalytic site functioning in antigen cleavage by H34 catalytic antibody light chain.Sci Rep. 2022 Nov 10;12(1):19185. doi: 10.1038/s41598-022-23689-6. Sci Rep. 2022. PMID: 36357546 Free PMC article.
-
Finding and characterizing a catalytic antibody light chain, H34, capable of degrading the PD-1 molecule.RSC Chem Biol. 2020 Dec 10;2(1):220-229. doi: 10.1039/d0cb00155d. eCollection 2021 Feb 1. RSC Chem Biol. 2020. PMID: 34458785 Free PMC article.
-
Catalytic antibodies in patients with systemic lupus erythematosus.Eur J Rheumatol. 2018 Sep;5(3):173-178. doi: 10.5152/eurjrheum.2018.17194. Epub 2018 Aug 7. Eur J Rheumatol. 2018. PMID: 30185370 Free PMC article.
-
Enzymatization of mouse monoclonal antibodies to the corresponding catalytic antibodies.Sci Rep. 2024 May 28;14(1):12184. doi: 10.1038/s41598-024-63116-6. Sci Rep. 2024. PMID: 38806597 Free PMC article.
References
-
- Mei S., Mody B., Eklund S. H., Paul S. (1991) Vasoactive intestinal peptide hydrolysis by antibody light chains. J. Biol. Chem. 266, 15571–15574 - PubMed
-
- Shuster A. M., Gololobov G. V., Kvashuk O. A., Bogomolova A. E., Smirnov I. V., Gabibov A. G. (1992) DNA hydrolyzing autoantibodies. Science 256, 665–667 - PubMed
-
- Hifumi E., Mitsuda Y., Ohara K., Uda T. (2002) Targeted destruction of the HIV-1 coat protein gp41 by a catalytic antibody light chain. J. Immunol. Methods 269, 283–298 - PubMed
-
- Paul S., Karle S., Planque S., Taguchi H., Salas M., Nishiyama Y., Handy B., Hunter R., Edmundson A., Hanson C. (2004) Naturally occurring proteolytic antibodies. Selective immunoglobulin M-catalyzed hydrolysis of HIV gp120. J. Biol. Chem. 279, 39611–39619 - PubMed
-
- Lacroix-Desmazes S., Moreau A., Sooryanarayana, Bonnemain C., Stieltjes N., Pashov A., Sultan Y., Hoebeke J., Kazatchkine M. D., Kaveri S. V. (1999) Catalytic activity of antibodies against factor VIII in patients with hemophilia A. Nat. Med. 5, 1044–1047 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

