Phase shift in the 24-hour rhythm of hippocampal EEG spiking activity in a rat model of temporal lobe epilepsy
- PMID: 23678009
- PMCID: PMC3763091
- DOI: 10.1152/jn.00911.2012
Phase shift in the 24-hour rhythm of hippocampal EEG spiking activity in a rat model of temporal lobe epilepsy
Abstract
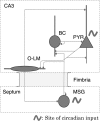
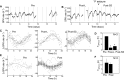
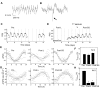
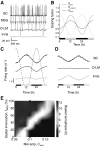
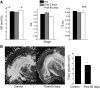
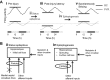
For over a century epileptic seizures have been known to cluster at specific times of the day. Recent studies have suggested that the circadian regulatory system may become permanently altered in epilepsy, but little is known about how this affects neural activity and the daily pattern of seizures. To investigate, we tracked long-term changes in the rate of spontaneous hippocampal EEG spikes (SPKs) in a rat model of temporal lobe epilepsy. In healthy animals, SPKs oscillated with near 24-h period; however, after injury by status epilepticus, a persistent phase shift of ∼12 h emerged in animals that later went on to develop chronic spontaneous seizures. Additional measurements showed that global 24-h rhythms, including core body temperature and theta state transitions, did not phase shift. Instead, we hypothesized that locally impaired circadian input to the hippocampus might be responsible for the SPK phase shift. This was investigated with a biophysical computer model in which we showed that subtle changes in the relative strengths of circadian input could produce a phase shift in hippocampal neural activity. MRI provided evidence that the medial septum, a putative circadian relay center for the hippocampus, exhibits signs of damage and therefore could contribute to local circadian impairment. Our results suggest that balanced circadian input is critical to maintaining natural circadian phase in the hippocampus and that damage to circadian relay centers, such as the medial septum, may disrupt this balance. We conclude by discussing how abnormal circadian regulation may contribute to the daily rhythms of epileptic seizures and related cognitive dysfunction.
Keywords: MRI; circadian input; epilepsy; hippocampus; in vivo; mathematical model; medial septum; sharp wave; status epilepticus.
Figures



References
-
- Assaf SY, Miller JJ. The role of a raphe serotonin system in the control of septal unit activity and hippocampal desynchronization. Neuroscience 3: 539–550, 1978 - PubMed
-
- Barnes CA, McNaughton BL, Goddard GV, Douglas RM, Adamec R. Circadian rhythm of synaptic excitability in rat and monkey central nervous system. Science 197: 91–92, 1977 - PubMed
-
- Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed 8: 333–344, 1995 - PubMed
-
- Bastlund JF, Jennum P, Mohapel P, Penschuck S, Watson WP. Spontaneous epileptic rats show changes in sleep architecture and hypothalamic pathology. Epilepsia 46: 934–938, 2005 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

