Genetic reconstruction of dopamine D1 receptor signaling in the nucleus accumbens facilitates natural and drug reward responses
- PMID: 23678109
- PMCID: PMC3684445
- DOI: 10.1523/JNEUROSCI.5532-12.2013
Genetic reconstruction of dopamine D1 receptor signaling in the nucleus accumbens facilitates natural and drug reward responses
Abstract
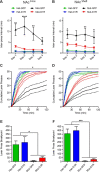
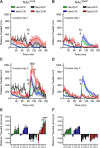
The dopamine D1 receptor (D1R) facilitates reward acquisition and its alteration leads to profound learning deficits. However, its minimal functional circuit requirement is unknown. Using conditional reconstruction of functional D1R signaling in D1R knock-out mice, we define distinct requirements of D1R in subregions of the nucleus accumbens (NAc) for specific dimensions of reward. We demonstrate that D1R expression in the core region of the NAc (NAc(Core)), but not the shell (NAc(Shell)), enhances selectively a unique form of pavlovian conditioned approach and mediates D1R-dependent cocaine sensitization. However, D1R expression in either the NAc(Core) or the NAc(Shell) improves instrumental responding for reward. In contrast, neither NAc(Core) nor NAc(Shell) D1R is sufficient to promote motivation to work for reward in a progressive ratio task or for motor learning. These results highlight dissociated circuit requirements of D1R for dopamine-dependent behaviors.
Figures




References
-
- Anderson SM, Bari AA, Pierce RC. Administration of the D1-like dopamine receptor antagonist SCH-23390 into the medial nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug-seeking behavior in rats. Psychopharmacology. 2003;168:132–138. doi: 10.1007/s00213-002-1298-5. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
