Suppression of serotonin neuron firing increases aggression in mice
- PMID: 23678112
- PMCID: PMC6618819
- DOI: 10.1523/JNEUROSCI.2067-12.2013
Suppression of serotonin neuron firing increases aggression in mice
Abstract
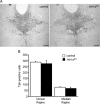
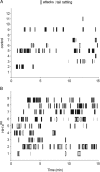
Numerous studies link decreased serotonin metabolites with increased impulsive and aggressive traits. However, although pharmacological depletion of serotonin is associated with increased aggression, interventions aimed at directly decreasing serotonin neuron activity have supported the opposite association. Furthermore, it is not clear if altered serotonin activity during development may contribute to some of the observed associations. Here, we used two pharmacogenetic approaches in transgenic mice to selectively and reversibly reduce the firing of serotonin neurons in behaving animals. Conditional overexpression of the serotonin 1A receptor (Htr1a) in serotonin neurons showed that a chronic reduction in serotonin neuron firing was associated with heightened aggression. Overexpression of Htr1a in adulthood, but not during development, was sufficient to increase aggression. Rapid suppression of serotonin neuron firing by agonist treatment of mice expressing Htr1a exclusively in serotonin neurons also led to increased aggression. These data confirm a role of serotonin activity in setting thresholds for aggressive behavior and support a direct association between low levels of serotonin homeostasis and increased aggression.
Figures






Similar articles
-
Sex hormone-dependent desensitization of 5-HT1A autoreceptors in knockout mice deficient in the 5-HT transporter.Eur J Neurosci. 2003 Oct;18(8):2203-12. doi: 10.1046/j.1460-9568.2003.02960.x. Eur J Neurosci. 2003. PMID: 14622181
-
Pet-1 is required across different stages of life to regulate serotonergic function.Nat Neurosci. 2010 Oct;13(10):1190-8. doi: 10.1038/nn.2623. Epub 2010 Sep 5. Nat Neurosci. 2010. PMID: 20818386 Free PMC article.
-
Serotonin 1A receptors alter expression of movement representations.J Neurosci. 2013 Mar 13;33(11):4988-99. doi: 10.1523/JNEUROSCI.4241-12.2013. J Neurosci. 2013. PMID: 23486969 Free PMC article.
-
5-HT1A Receptor-Mediated Autoinhibition and the Control of Serotonergic Cell Firing.ACS Chem Neurosci. 2015 Jul 15;6(7):1110-5. doi: 10.1021/acschemneuro.5b00034. Epub 2015 May 26. ACS Chem Neurosci. 2015. PMID: 25913021 Free PMC article. Review.
-
5-HT1A and 5-HT1B receptor agonists and aggression: a pharmacological challenge of the serotonin deficiency hypothesis.Eur J Pharmacol. 2005 Dec 5;526(1-3):125-39. doi: 10.1016/j.ejphar.2005.09.065. Epub 2005 Nov 28. Eur J Pharmacol. 2005. PMID: 16310183 Review.
Cited by
-
Negative Influence of the Hunger State on Rule-observance Behavior in Mice.Exp Neurobiol. 2023 Feb 28;32(1):31-41. doi: 10.5607/en22036. Exp Neurobiol. 2023. PMID: 36919334 Free PMC article.
-
Dietary L-tryptophan modulates agonistic behavior and brain serotonin in male dyadic contests of a cichlid fish.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2019 Dec;205(6):867-880. doi: 10.1007/s00359-019-01373-x. Epub 2019 Nov 5. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2019. PMID: 31691094
-
Deletion of the P/Q-Type Calcium Channel from Serotonergic Neurons Drives Male Aggression in Mice.J Neurosci. 2022 Aug 24;42(34):6637-6653. doi: 10.1523/JNEUROSCI.0204-22.2022. J Neurosci. 2022. PMID: 35853721 Free PMC article.
-
Embracing diversity in the 5-HT neuronal system.Nat Rev Neurosci. 2019 Jul;20(7):397-424. doi: 10.1038/s41583-019-0151-3. Nat Rev Neurosci. 2019. PMID: 30948838 Review.
-
Interacting effect of MAOA genotype and maternal prenatal smoking on aggressive behavior in young adulthood.J Neural Transm (Vienna). 2016 Aug;123(8):885-94. doi: 10.1007/s00702-016-1582-x. Epub 2016 Jun 14. J Neural Transm (Vienna). 2016. PMID: 27300740
References
-
- Bonnavion P, Bernard JF, Hamon M, Adrien J, Fabre V. Heterogeneous distribution of the serotonin 5-HT(1A) receptor mRNA in chemically identified neurons of the mouse rostral brainstem: Implications for the role of serotonin in the regulation of wakefulness and REM sleep. J Comp Neurol. 2010;518:2744–2770. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
