The synchronous activity of lateral habenular neurons is essential for regulating hippocampal theta oscillation
- PMID: 23678132
- PMCID: PMC6618841
- DOI: 10.1523/JNEUROSCI.4369-12.2013
The synchronous activity of lateral habenular neurons is essential for regulating hippocampal theta oscillation
Abstract
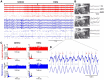
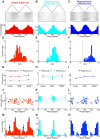
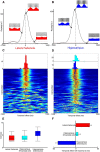
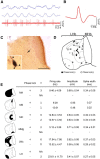
Lateral habenula (LHb) has attracted growing interest as a regulator of serotonergic and dopaminergic neurons in the CNS. However, it remains unclear how the LHb modulates brain states in animals. To identify the neural substrates that are under the influence of LHb regulation, we examined the effects of rat LHb lesions on the hippocampal oscillatory activity associated with the transition of brain states. Our results showed that the LHb lesion shortened the theta activity duration both in anesthetized and sleeping rats. Furthermore, this inhibitory effect of LHb lesion on theta maintenance depended upon an intact serotonergic median raphe, suggesting that LHb activity plays an essential role in maintaining hippocampal theta oscillation via the serotonergic raphe. Multiunit recording of sleeping rats further revealed that firing of LHb neurons showed significant phase-locking activity at each theta oscillation cycle in the hippocampus. LHb neurons showing activity that was coordinated with that of the hippocampal theta were localized in the medial LHb division, which receives afferents from the diagonal band of Broca (DBB), a pacemaker region for the hippocampal theta oscillation. Thus, our findings indicate that the DBB may pace not only the hippocampus, but also the LHb, during rapid eye movement sleep. Since serotonin is known to negatively regulate theta oscillation in the hippocampus, phase-locking activity of the LHb neurons may act, under the influence of the DBB, to maintain the hippocampal theta oscillation by modulating the activity of serotonergic neurons.
Conflict of interest statement
The authors declare no conflict of interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
