Novel inhibitors of severe acute respiratory syndrome coronavirus entry that act by three distinct mechanisms
- PMID: 23678171
- PMCID: PMC3700180
- DOI: 10.1128/JVI.00998-13
Novel inhibitors of severe acute respiratory syndrome coronavirus entry that act by three distinct mechanisms
Abstract
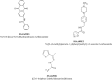
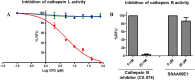
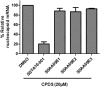
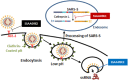
Severe acute respiratory syndrome (SARS) is an infectious and highly contagious disease that is caused by SARS coronavirus (SARS-CoV) and for which there are currently no approved treatments. We report the discovery and characterization of small-molecule inhibitors of SARS-CoV replication that block viral entry by three different mechanisms. The compounds were discovered by screening a chemical library of compounds for blocking of entry of HIV-1 pseudotyped with SARS-CoV surface glycoprotein S (SARS-S) but not that of HIV-1 pseudotyped with vesicular stomatitis virus surface glycoprotein G (VSV-G). Studies on their mechanisms of action revealed that the compounds act by three distinct mechanisms: (i) SSAA09E2 {N-[[4-(4-methylpiperazin-1-yl)phenyl]methyl]-1,2-oxazole-5-carboxamide} acts through a novel mechanism of action, by blocking early interactions of SARS-S with the receptor for SARS-CoV, angiotensin converting enzyme 2 (ACE2); (ii) SSAA09E1 {[(Z)-1-thiophen-2-ylethylideneamino]thiourea} acts later, by blocking cathepsin L, a host protease required for processing of SARS-S during viral entry; and (iii) SSAA09E3 [N-(9,10-dioxo-9,10-dihydroanthracen-2-yl)benzamide] also acts later and does not affect interactions of SARS-S with ACE2 or the enzymatic functions of cathepsin L but prevents fusion of the viral membrane with the host cellular membrane. Our work demonstrates that there are at least three independent strategies for blocking SARS-CoV entry, validates these mechanisms of inhibition, and introduces promising leads for the development of SARS therapeutics.
Figures



References
-
- Poutanen SM, Low DE, Henry B, Finkelstein S, Rose D, Green K, Tellier R, Draker R, Adachi D, Ayers M, Chan AK, Skowronski DM, Salit I, Simor AE, Slutsky AS, Doyle PW, Krajden M, Petric M, Brunham RC, McGeer AJ. 2003. Identification of severe acute respiratory syndrome in Canada. N. Engl. J. Med. 348:1995–2005 - PubMed
-
- Booth CM, Matukas LM, Tomlinson GA, Rachlis AR, Rose DB, Dwosh HA, Walmsley SL, Mazzulli T, Avendano M, Derkach P, Ephtimios IE, Kitai I, Mederski BD, Shadowitz SB, Gold WL, Hawryluck LA, Rea E, Chenkin JS, Cescon DW, Poutanen SM, Detsky AS. 2003. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA 289:2801–2809 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

