Molecular determinants of hepatitis B and D virus entry restriction in mouse sodium taurocholate cotransporting polypeptide
- PMID: 23678176
- PMCID: PMC3700185
- DOI: 10.1128/JVI.03540-12
Molecular determinants of hepatitis B and D virus entry restriction in mouse sodium taurocholate cotransporting polypeptide
Erratum in
-
Correction for Yan et al., "Molecular Determinants of Hepatitis B and D Virus Entry Restriction in Mouse Sodium Taurocholate Cotransporting Polypeptide".J Virol. 2025 May 20;99(5):e0044525. doi: 10.1128/jvi.00445-25. Epub 2025 Apr 16. J Virol. 2025. PMID: 40237500 Free PMC article. No abstract available.
Abstract
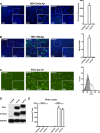
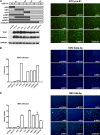
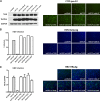
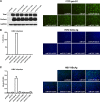
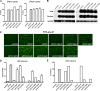
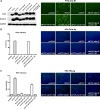
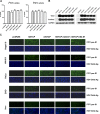
Human hepatitis B virus (HBV) and its satellite virus, hepatitis D virus (HDV), primarily infect humans, chimpanzees, or tree shrews (Tupaia belangeri). Viral infections in other species are known to be mainly restricted at the entry level since viral replication can be achieved in the cells by transfection of the viral genome. Sodium taurocholate cotransporting polypeptide (NTCP) is a functional receptor for HBV and HDV, and amino acids 157 to 165 of NTCP are critical for viral entry and likely limit viral infection of macaques. However, the molecular determinants for viral entry restriction in mouse NTCP (mNTCP) remain unclear. In this study, mNTCP was found to be unable to support either HBV or HDV infection, although it can bind to pre-S1 of HBV L protein and is functional in transporting substrate taurocholate; comprehensive swapping and point mutations of human NTCP (hNTCP) and mNTCP revealed molecular determinants restricting mNTCP for viral entry of HBV and HDV. Remarkably, when mNTCP residues 84 to 87 were substituted by human counterparts, mNTCP can effectively support viral infections. In addition, a number of cell lines, regardless of their species or tissue origin, supported HDV infection when transfected with hNTCP or mNTCP with residues 84 to 87 replaced by human counterparts, highlighting the central role of NTCP for viral infections mediated by HBV envelope proteins. These studies advance our understanding of NTCP-mediated viral entry of HBV and HDV and have important implications for developing the mouse model for their infections.
Figures


References
-
- Ganem D, Prince AM. 2004. Hepatitis B virus infection: natural history and clinical consequences. N. Engl. J. Med. 350:1118–1129 - PubMed
-
- Lavanchy D. 2004. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J. Viral Hepat. 11:97–107 - PubMed
-
- Chan HL, Sung JJ. 2006. Hepatocellular carcinoma and hepatitis B virus. Semin. Liver Dis. 26:153–161 - PubMed
-
- Hughes SA, Wedemeyer H, Harrison PM. 2011. Hepatitis delta virus. Lancet 378:73–85 - PubMed
-
- Nassal M. 1996. Hepatitis B virus morphogenesis. Curr. Top. Microbiol. Immunol. 214:297–337 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

