Usefulness of applying lidocaine in esophagogastroduodenoscopy performed under sedation with propofol
- PMID: 23678376
- PMCID: PMC3653022
- DOI: 10.4253/wjge.v5.i5.231
Usefulness of applying lidocaine in esophagogastroduodenoscopy performed under sedation with propofol
Abstract
Aim: To determine whether topical lidocaine benefits esophagogastroduoduenoscopy (EGD) by decreasing propofol dose necessary for sedation or procedure-related complications.
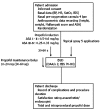
Methods: The study was designed as a prospective, single centre, double blind, randomised clinical trial and was conducted in 2012 between January and May (NCT01489891). Consecutive patients undergoing EGD were randomly assigned to receive supplemental topical lidocaine (L; 50 mg in an excipient solution which was applied as a spray to the oropharynx) or placebo (P; taste excipients solution without active substance, similarly delivered) prior to the standard propofol sedation procedure. The propofol was administered as a bolus intravenous (iv) dose, with patients in the L and P groups receiving initial doses based on the patient's American Society of Anaesthesiologists (ASA) classification (ASA I-II: 0.50-0.60 mg/kg; ASA III-IV: 0.25-0.35 mg/kg), followed by 10-20 mg iv dose every 30-60 s at the anaesthetist's discretion. Vital signs, anthropometric measurements, amount of propofol administered, sedation level reached, examination time, and the subjective assessments of the endoscopist's and anaesthetist's satisfaction (based upon a four point Likert scale) were recorded. All statistical tests were performed by the Stata statistical software suite (Release 11, 2009; StataCorp, LP, College Station, TX, United States).
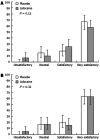
Results: No significant differences were found between the groups treated with lidocaine or placebo in terms of total propofol dose (310.7 ± 139.2 mg/kg per minute vs 280.1 ± 87.7 mg/kg per minute, P = 0.15) or intraprocedural propofol dose (135.3 ± 151.7 mg/kg per minute vs 122.7 ± 96.5 mg/kg per minute, P = 0.58). Only when the L and P groups were analysed with the particular subgroups of female, < 65-year-old, and lower anaesthetic risk level (ASA I-II) was a statistically significant difference found (L: 336.5 ± 141.2 mg/kg per minute vs P: 284.6 ± 91.2 mg/kg per minute, P = 0.03) for greater total propofol requirements). The total incidence of complications was also similar between the two groups, with the L group showing a complication rate of 32.2% (95%CI: 21.6-45.0) and the P group showing a complication rate of 26.7% (95%CI: 17.0-39.0). In addition, the use of lidocaine had no effect on the anaesthetist's or endoscopist's satisfaction with the procedure. Thus, the endoscopist's satisfaction Likert assessments were equally distributed among the L and P groups: unsatisfactory, [L: 6.8% (95%CI: 2.2-15.5) vs P: 0% (95%CI: 0-4.8); neutral, L: 10.1% (95%CI: 4.2-19.9) vs P: 15% (95%CI: 7.6-25.7)]; satisfactory, [L: 25.4% (95%CI: 10-29.6) vs P: 18.3% (95%CI: 15.5-37.6); and very satisfactory, L: 57.6% (95%CI: 54-77.7) vs P: 66.6% (95%CI: 44.8-69.7)]. Likewise, the anaesthetist's satisfaction Likert assessments regarding the ease of maintaining a patient at an optimum sedation level without agitation or modification of the projected sedation protocol were not affected by the application of lidocaine, as evidenced by the lack of significant differences between the scores for the placebo group: unsatisfactory, L: 5.8% (95%CI: 1.3-13.2) vs P: 0% (95%CI: 0-4.8); neutral, L: 16.9% (95%CI: 8.9-28.4) vs P: 16.7% (95%CI: 8.8-27.7); satisfactory, L: 15.2% (95%CI: 7.7-26.1) vs P: 20.3% (95%CI: 11.3-31.6); and very satisfactory, L: 62.7% (95%CI: 49.9-74.3) vs P: 63.3% (95%CI: 50.6-74.7).
Conclusion: Topical pharyngeal anaesthesia is safe in EGD but does not reduce the necessary dose of propofol or improve the anaesthetist's or endoscopist's satisfaction with the procedure.
Keywords: Adverse effects; Esophagogastroduodenoscopy; Lidocaine; Propofol; Sedation.
Figures
References
-
- Heuss LT, Froehlich F, Beglinger C. Changing patterns of sedation and monitoring practice during endoscopy: results of a nationwide survey in Switzerland. Endoscopy. 2005;37:161–166. - PubMed
-
- Rex DK, Deenadayalu VP, Eid E, Imperiale TF, Walker JA, Sandhu K, Clarke AC, Hillman LC, Horiuchi A, Cohen LB, et al. Endoscopist-directed administration of propofol: a worldwide safety experience. Gastroenterology. 2009;137:1229–137; quiz 1229-137;. - PubMed
-
- Riphaus A, Rabofski M, Wehrmann T. Endoscopic sedation and monitoring practice in Germany: results from the first nationwide survey. Z Gastroenterol. 2010;48:392–397. - PubMed
-
- Thomson A, Andrew G, Jones DB. Optimal sedation for gastrointestinal endoscopy: review and recommendations. J Gastroenterol Hepatol. 2010;25:469–478. - PubMed
-
- Goulson DT, Fragneto RY. Anesthesia for gastrointestinal endoscopic procedures. Anesthesiol Clin. 2009;27:71–85. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

