GenK-catalyzed C-6' methylation in the biosynthesis of gentamicin: isolation and characterization of a cobalamin-dependent radical SAM enzyme
- PMID: 23679096
- PMCID: PMC3796153
- DOI: 10.1021/ja312641f
GenK-catalyzed C-6' methylation in the biosynthesis of gentamicin: isolation and characterization of a cobalamin-dependent radical SAM enzyme
Abstract
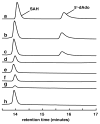
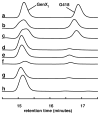
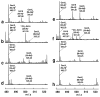
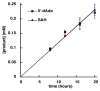
The existence of cobalamin (Cbl)-dependent enzymes that are members of the radical S-adenosyl-l-methionine (SAM) superfamily was previously predicted on the basis of bioinformatic analysis. A number of these are Cbl-dependent methyltransferases, but the details surrounding their reaction mechanisms have remained unclear. In this report we demonstrate the in vitro activity of GenK, a Cbl-dependent radical SAM enzyme that methylates an unactivated sp(3) carbon during the biosynthesis of gentamicin, an aminoglycoside antibiotic. Experiments to investigate the stoichiometry of the GenK reaction revealed that 1 equiv each of 5'-deoxyadenosine and S-adenosyl-homocysteine are produced for each methylation reaction catalyzed by GenK. Furthermore, isotope-labeling experiments demonstrate that the S-methyl group from SAM is transferred to Cbl and the aminoglycoside product during the course of the reaction. On the basis of these results, one mechanistic possibility for the GenK reaction can be ruled out, and further questions regarding the mechanisms of Cbl-dependent radical SAM methyltransferases, in general, are discussed.
Figures


Similar articles
-
Reaction Catalyzed by GenK, a Cobalamin-Dependent Radical S-Adenosyl-l-methionine Methyltransferase in the Biosynthetic Pathway of Gentamicin, Proceeds with Retention of Configuration.J Am Chem Soc. 2017 Nov 15;139(45):16084-16087. doi: 10.1021/jacs.7b09890. Epub 2017 Nov 7. J Am Chem Soc. 2017. PMID: 29091410 Free PMC article.
-
Biosynthesis of Branched Alkoxy Groups: Iterative Methyl Group Alkylation by a Cobalamin-Dependent Radical SAM Enzyme.J Am Chem Soc. 2017 Feb 8;139(5):1742-1745. doi: 10.1021/jacs.6b10901. Epub 2017 Jan 25. J Am Chem Soc. 2017. PMID: 28040895 Free PMC article.
-
The B12-Radical SAM Enzyme PoyC Catalyzes Valine Cβ-Methylation during Polytheonamide Biosynthesis.J Am Chem Soc. 2016 Dec 7;138(48):15515-15518. doi: 10.1021/jacs.6b06697. Epub 2016 Nov 29. J Am Chem Soc. 2016. PMID: 27934015 Free PMC article.
-
Cobalamin-dependent radical S-adenosyl-l-methionine enzymes in natural product biosynthesis.Nat Prod Rep. 2018 Aug 15;35(8):707-720. doi: 10.1039/c7np00059f. Nat Prod Rep. 2018. PMID: 30079906 Review.
-
Radical-mediated enzymatic methylation: a tale of two SAMS.Acc Chem Res. 2012 Apr 17;45(4):555-64. doi: 10.1021/ar200202c. Epub 2011 Nov 18. Acc Chem Res. 2012. PMID: 22097883 Free PMC article. Review.
Cited by
-
Changing Fates of the Substrate Radicals Generated in the Active Sites of the B12-Dependent Radical SAM Enzymes OxsB and AlsB.J Am Chem Soc. 2023 Feb 15;145(6):3656-3664. doi: 10.1021/jacs.2c12953. Epub 2023 Jan 31. J Am Chem Soc. 2023. PMID: 36719327 Free PMC article.
-
Eukaryotic TYW1 Is a Radical SAM Flavoenzyme.Biochemistry. 2021 Jul 13;60(27):2179-2185. doi: 10.1021/acs.biochem.1c00349. Epub 2021 Jun 29. Biochemistry. 2021. PMID: 34184886 Free PMC article.
-
Characterization and utilization of methyltransferase for apramycin production in Streptoalloteichus tenebrarius.J Ind Microbiol Biotechnol. 2022 Jul 30;49(4):kuac011. doi: 10.1093/jimb/kuac011. J Ind Microbiol Biotechnol. 2022. PMID: 35536571 Free PMC article.
-
The intriguing biology and chemistry of fosfomycin: the only marketed phosphonate antibiotic.RSC Adv. 2019 Dec 19;9(72):42204-42218. doi: 10.1039/c9ra08299a. eCollection 2019 Dec 18. RSC Adv. 2019. PMID: 35548698 Free PMC article. Review.
-
Spectroscopic characterization and mechanistic investigation of P-methyl transfer by a radical SAM enzyme from the marine bacterium Shewanella denitrificans OS217.Biochim Biophys Acta. 2014 Dec;1844(12):2135-44. doi: 10.1016/j.bbapap.2014.09.009. Epub 2014 Sep 16. Biochim Biophys Acta. 2014. PMID: 25224746 Free PMC article.
References
-
- Frey PA, Hegeman AD, Ruzicka FJ. Crit Rev Biochem Mol Biol. 2008;43:63–88. - PubMed
-
- Shepard EM, Broderick JB. In: Comprehensive Natural Products II, Chemistry and Biology. Mander L, Liu Hw, editors. Vol. 8. Elsevier; Oxford: 2010. pp. 625–661.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

