Basal forebrain activation controls contrast sensitivity in primary visual cortex
- PMID: 23679191
- PMCID: PMC3662585
- DOI: 10.1186/1471-2202-14-55
Basal forebrain activation controls contrast sensitivity in primary visual cortex
Abstract
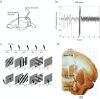
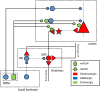
Background: The basal forebrain (BF) regulates cortical activity by the action of cholinergic projections to the cortex. At the same time, it also sends substantial GABAergic projections to both cortex and thalamus, whose functional role has received far less attention. We used deep brain stimulation (DBS) in the BF, which is thought to activate both types of projections, to investigate the impact of BF activation on V1 neural activity.
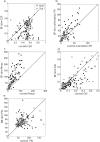
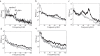
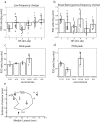
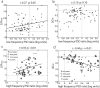
Results: BF stimulation robustly increased V1 single and multi-unit activity, led to moderate decreases in orientation selectivity and a remarkable increase in contrast sensitivity as demonstrated by a reduced semi-saturation contrast. The spontaneous V1 local field potential often exhibited spectral peaks centered at 40 and 70 Hz as well as reliably showed a broad γ-band (30-90 Hz) increase following BF stimulation, whereas effects in a low frequency band (1-10 Hz) were less consistent. The broad γ-band, rather than low frequency activity or spectral peaks was the best predictor of both the firing rate increase and contrast sensitivity increase of V1 unit activity.
Conclusions: We conclude that BF activation has a strong influence on contrast sensitivity in V1. We suggest that, in addition to cholinergic modulation, the BF GABAergic projections play a crucial role in the impact of BF DBS on cortical activity.
Figures

References
-
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983;214(2):170–197. doi: 10.1002/cne.902140206. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

