Conditionally replicative adenoviral vectors for imaging the effect of chemotherapy on pancreatic cancer cells
- PMID: 23679574
- PMCID: PMC7657248
- DOI: 10.1111/cas.12196
Conditionally replicative adenoviral vectors for imaging the effect of chemotherapy on pancreatic cancer cells
Abstract
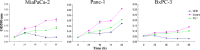
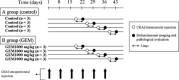
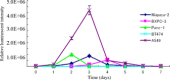
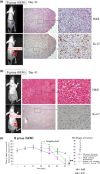
Pancreatic cancer has a poor prognosis after complete macroscopic resection combined with chemotherapy. Even after neoadjuvant chemotherapy, R0 resection is often not possible. Moreover, current imaging techniques cannot reliably distinguish viable cancer cells from scar tissue at the resectional margin. We investigated the use of a conditionally replicative adenovirus (CRAd), Ad5/3Cox2CRAd-ΔE3ADP-Luc, for imaging the effects of chemotherapy. The CRAd infectivity of pancreatic cancer cells was enhanced by a chimeric Ad5/3 fiber, E1A expression was under the control of the Cox2 promoter, and the luciferase gene was inserted adjacent to the adenovirus death protein (ADP) gene. Subcutaneous xenografts of the pancreatic cancer cell line MiaPaCa-2 were established in 24 BALB/c nu/nu mice. When xenografts reached a diameter of 4-6 mm (day 1), the mice were injected i.p. with either PBS (group A; n = 12) or 1000 mg/kg gemcitabine (group B; n = 12), weekly. On days 19, 26, 33, and 40, CRAd were injected intratumorally into three mice in groups A and B. Bioluminescence was imaged 72 h after CRAd injection, and gross tumor volumes were measured then tumors were removed for ex vivo histopathology using H&E and Ki-67 staining. Correlations between gross tumor volume, pathological evaluation of the percentage of viable tumor area, and CRAd bioluminescence were analyzed. Bioluminescence correlated closely with the percentage of viable tumor area (R = 0.96), but not with gross tumor volume (R = 0.31). Therefore, CRAds might be reliable imaging tools for monitoring chemotherapy in pancreatic cancer, and could improve our ability to distinguish viable tumor cells from scar tissue.
© 2013 Japanese Cancer Association.
Figures
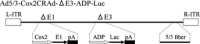


Similar articles
-
Combination with low-dose gemcitabine and hTERT-promoter-dependent conditionally replicative adenovirus enhances cytotoxicity through their crosstalk mechanisms in pancreatic cancer.Cancer Lett. 2010 Aug 28;294(2):178-86. doi: 10.1016/j.canlet.2010.01.034. Epub 2010 Feb 16. Cancer Lett. 2010. PMID: 20163915
-
Biodistribution and safety assessment of bladder cancer specific recombinant oncolytic adenovirus in subcutaneous xenografts tumor model in nude mice.Curr Gene Ther. 2012 Apr 1;12(2):67-76. doi: 10.2174/156652312800099599. Curr Gene Ther. 2012. PMID: 22384806 Free PMC article.
-
Generation of a novel, cyclooxygenase-2-targeted, interferon-expressing, conditionally replicative adenovirus for pancreatic cancer therapy.Am J Surg. 2012 Nov;204(5):741-50. doi: 10.1016/j.amjsurg.2012.02.016. Epub 2012 Jun 29. Am J Surg. 2012. PMID: 22748294 Free PMC article.
-
Combining high selectivity of replication via CXCR4 promoter with fiber chimerism for effective adenoviral oncolysis in breast cancer.Int J Cancer. 2007 Feb 15;120(4):935-41. doi: 10.1002/ijc.22338. Int J Cancer. 2007. PMID: 17131341
-
Engineering regulatory elements for conditionally-replicative adeno-viruses.Curr Gene Ther. 2003 Aug;3(4):357-85. doi: 10.2174/1566523034578311. Curr Gene Ther. 2003. PMID: 12871022 Review.
Cited by
-
A novel capsid-modified oncolytic recombinant adenovirus type 5 for tumor-targeting gene therapy by intravenous route.Oncotarget. 2016 Jul 26;7(30):47287-47301. doi: 10.18632/oncotarget.10075. Oncotarget. 2016. PMID: 27323824 Free PMC article.
-
Targeting Tumor-Associated Fibroblasts for Therapeutic Delivery in Desmoplastic Tumors.Cancer Res. 2017 Feb 1;77(3):719-731. doi: 10.1158/0008-5472.CAN-16-0866. Epub 2016 Nov 18. Cancer Res. 2017. PMID: 27864344 Free PMC article.
-
CIK cell-based delivery of recombinant adenovirus KGHV500 carrying the anti-p21Ras scFv gene enhances the anti-tumor effect and safety in lung cancer.J Cancer Res Clin Oncol. 2019 May;145(5):1123-1132. doi: 10.1007/s00432-019-02857-8. Epub 2019 Feb 22. J Cancer Res Clin Oncol. 2019. PMID: 30796510 Free PMC article.
References
-
- Jemal A, Siegel R, Ward E et al Cancer statistics, 2008. CA Cancer J Clin 2008; 58: 71–96. - PubMed
-
- Neoptolemos JP, Stocken DD, Friess H et al A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004; 350: 1200–10. - PubMed
-
- Neoptolemos JP, Stocken DD, Bassi C et al Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA 2010; 304: 1073–81. - PubMed
-
- Oettle H, Post S, Neuhaus P et al Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative‐intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007; 297: 267–77. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

