D1-D2 dopamine receptor synergy promotes calcium signaling via multiple mechanisms
- PMID: 23680635
- PMCID: PMC3716318
- DOI: 10.1124/mol.113.085175
D1-D2 dopamine receptor synergy promotes calcium signaling via multiple mechanisms
Abstract
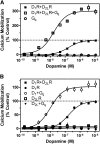
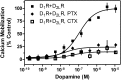
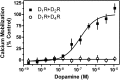
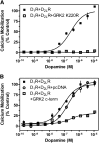
The D(1) dopamine receptor (D(1)R) has been proposed to form a hetero-oligomer with the D(2) dopamine receptor (D(2)R), which in turn results in a complex that couples to phospholipase C-mediated intracellular calcium release. We have sought to elucidate the pharmacology and mechanism of action of this putative signaling pathway. Dopamine dose-response curves assaying intracellular calcium mobilization in cells heterologously expressing the D(1) and D(2) subtypes, either alone or in combination, and using subtype selective ligands revealed that concurrent stimulation is required for coupling. Surprisingly, characterization of a putative D(1)-D(2) heteromer-selective ligand, 6-chloro-2,3,4,5-tetrahydro-3-methyl-1-(3-methylphenyl)-1H-3-benzazepine-7,8-diol (SKF83959), found no stimulation of calcium release, but it did find a broad range of cross-reactivity with other G protein-coupled receptors. In contrast, SKF83959 appeared to be an antagonist of calcium mobilization. Overexpression of G(qα) with the D(1) and D(2) dopamine receptors enhanced the dopamine-stimulated calcium response. However, this was also observed in cells expressing G(qα) with only the D1R. Inactivation of Gi or Gs with pertussis or cholera toxin, respectively, largely, but not entirely, reduced the calcium response in D(1)R and D(2)R cotransfected cells. Moreover, sequestration of G(βγ) subunits through overexpression of G protein receptor kinase 2 mutants either completely or largely eliminated dopamine-stimulated calcium mobilization. Our data suggest that the mechanism of D(1)R/D(2)R-mediated calcium signaling involves more than receptor-mediated G(q) protein activation, may largely involve downstream signaling pathways, and may not be completely heteromer-specific. In addition, SKF83959 may not exhibit selective activation of D(1)-D(2) heteromers, and its significant cross-reactivity to other receptors warrants careful interpretation of its use in vivo.
Figures






References
-
- Aizman O, Brismar H, Uhlén P, Zettergren E, Levey AI, Forssberg H, Greengard P, Aperia A. (2000) Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nat Neurosci 3:226–230 - PubMed
-
- Allen JA, Halverson-Tamboli RA, Rasenick MM. (2007) Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci 8:128–140 - PubMed
-
- Andringa G, Stoof JC, Cools AR. (1999) Sub-chronic administration of the dopamine D(1) antagonist SKF 83959 in bilaterally MPTP-treated rhesus monkeys: stable therapeutic effects and wearing-off dyskinesia. Psychopharmacology (Berl) 146:328–334 - PubMed
-
- Ariano MA, Larson ER, Noblett KL, Sibley DR, Levine MS. (1997) Coexpression of striatal dopamine receptor subtypes and excitatory amino acid subunits. Synapse 26:400–414 - PubMed
-
- Beaulieu J-M, Gainetdinov RR. (2011) The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63:182–217 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

