Do probiotics improve eradication response to Helicobacter pylori on standard triple or sequential therapy?
- PMID: 23680708
- PMCID: PMC3709373
- DOI: 10.4103/1319-3767.111953
Do probiotics improve eradication response to Helicobacter pylori on standard triple or sequential therapy?
Abstract
Background: The standard triple therapy for the eradication of Helicobacter pylori consists of a combination of a proton pump inhibitor at a standard dose together with two antibiotics (amoxicillin 1000 mg plus either clarithromycin 500 mg or metronidazole 400 mg) all given twice daily for a period of 7-14 days. Recent reports have shown a dramatic decline in the rate of H. pylori eradication utilizing standard triple therapy from 95% down to 70-80%.
Aims: Our study was designed to evaluate the effect of adding a probiotic as an adjuvant to common regimens used for H. pylori eradication.
Materials and methods: An open label randomized observational clinical study was designed to test three different regimens of H. pylori eradication treatment: Standard triple therapy with a concomitant probiotic added at the same time (n = 100), starting the probiotic for 2 weeks before initiating standard triple therapy along with the probiotic (n = 95), and the third regimen consists of the probiotic given concomitantly to sequential treatment (n = 76). The three arms were compared to a control group of patients treated with the traditional standard triple therapy (n = 106).
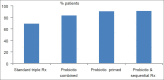
Results: The eradication rate for the traditional standard therapy was 68.9%, and adding the probiotic "Bifidus infantis" to triple therapy, led to a successful rate of eradication of 83% (P < 0.001). Pre-treatment with 2 weeks of B. infantis before adding it to standard triple therapy increased the success rate of eradication to 90.5%. Similar improvement in eradication rate was noted when B. infantis was added as an adjuvant to the sequential therapy leading to an eradication rate of 90.8%.
Conclusion: Adding B. infantis as an adjuvant to several therapeutic regimens commonly used for the eradication of H. pylori infection significantly improves the cure rates.
Conflict of interest statement
Figures
Comment in
-
Helicobacter pylori in the era of probiotics: a controversial application.Saudi J Gastroenterol. 2013 Sep-Oct;19(5):240-1. doi: 10.4103/1319-3767.118140. Saudi J Gastroenterol. 2013. PMID: 24045601 Free PMC article. No abstract available.
References
-
- Dajani AI, Awad S, Ukabam S, Nounou MA, Abdul Rasheed Z, Gautam S, et al. One-week triple regime therapy consisting of pantoprazole, amoxicillin and clarithromycin for cure of Helicobacter pylori-associated upper gastrointestinal diseases. Digestion. 1999;60:298–304. - PubMed
-
- Chey WD, Wong BC Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808–25. - PubMed
-
- Abou Hammour A, Dajani AI, Nounou M, Zakaria M, Mohammed N, Al Badri S. Current stand of classical triple therapy for the eradication of H. pylori in the UAE. Proceedings of the 6th Emirates Gastroenterology and Hepatology Conference, oral presentation in H. pylori plenary session. 2010 Apr
-
- Vaira D, Zullo A, Vakil N, Gatta L, Ricci C, Perna F, et al. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: A randomized trial. Ann Intern Med. 2007;146:556–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical