Plant defense against insect herbivores
- PMID: 23681010
- PMCID: PMC3676838
- DOI: 10.3390/ijms140510242
Plant defense against insect herbivores
Abstract
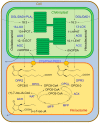
Plants have been interacting with insects for several hundred million years, leading to complex defense approaches against various insect feeding strategies. Some defenses are constitutive while others are induced, although the insecticidal defense compound or protein classes are often similar. Insect herbivory induce several internal signals from the wounded tissues, including calcium ion fluxes, phosphorylation cascades and systemic- and jasmonate signaling. These are perceived in undamaged tissues, which thereafter reinforce their defense by producing different, mostly low molecular weight, defense compounds. These bioactive specialized plant defense compounds may repel or intoxicate insects, while defense proteins often interfere with their digestion. Volatiles are released upon herbivory to repel herbivores, attract predators or for communication between leaves or plants, and to induce defense responses. Plants also apply morphological features like waxes, trichomes and latices to make the feeding more difficult for the insects. Extrafloral nectar, food bodies and nesting or refuge sites are produced to accommodate and feed the predators of the herbivores. Meanwhile, herbivorous insects have adapted to resist plant defenses, and in some cases even sequester the compounds and reuse them in their own defense. Both plant defense and insect adaptation involve metabolic costs, so most plant-insect interactions reach a stand-off, where both host and herbivore survive although their development is suboptimal.
Figures

Similar articles
-
[The role of volatile organic compounds in plant-insect communication].Biol Aujourdhui. 2024;218(3-4):141-144. doi: 10.1051/jbio/2024016. Epub 2025 Jan 27. Biol Aujourdhui. 2024. PMID: 39868713 Review. French.
-
A tritrophic signal that attracts parasitoids to host-damaged plants withstands disruption by non-host herbivores.BMC Plant Biol. 2010 Nov 15;10:247. doi: 10.1186/1471-2229-10-247. BMC Plant Biol. 2010. PMID: 21078181 Free PMC article.
-
Indirect plant defense against insect herbivores: a review.Insect Sci. 2018 Feb;25(1):2-23. doi: 10.1111/1744-7917.12436. Epub 2017 Mar 20. Insect Sci. 2018. PMID: 28035791 Review.
-
Mechanisms of plant defense against insect herbivores.Plant Signal Behav. 2012 Oct 1;7(10):1306-20. doi: 10.4161/psb.21663. Epub 2012 Aug 20. Plant Signal Behav. 2012. PMID: 22895106 Free PMC article. Review.
-
Plant immunity to insect herbivores.Annu Rev Plant Biol. 2008;59:41-66. doi: 10.1146/annurev.arplant.59.032607.092825. Annu Rev Plant Biol. 2008. PMID: 18031220 Review.
Cited by
-
Poplar protease inhibitor expression differs in an herbivore specific manner.BMC Plant Biol. 2021 Apr 9;21(1):170. doi: 10.1186/s12870-021-02936-4. BMC Plant Biol. 2021. PMID: 33836664 Free PMC article.
-
Differential Transcription and Alternative Splicing in Cotton Underly Specialized Defense Responses Against Pests.Front Plant Sci. 2020 Sep 15;11:573131. doi: 10.3389/fpls.2020.573131. eCollection 2020. Front Plant Sci. 2020. PMID: 33072149 Free PMC article.
-
Infestation of Rice Striped Stem Borer (Chilo suppressalis) Larvae Induces Emission of Volatile Organic Compounds in Rice and Repels Female Adult Oviposition.Int J Mol Sci. 2024 Aug 13;25(16):8827. doi: 10.3390/ijms25168827. Int J Mol Sci. 2024. PMID: 39201513 Free PMC article.
-
Benzothiadiazole and B-Aminobutyricacid Induce Resistance to Ectropis Obliqua in Tea Plants (Camellia Sinensis (L.) O. Kuntz).Molecules. 2018 May 28;23(6):1290. doi: 10.3390/molecules23061290. Molecules. 2018. PMID: 29843375 Free PMC article.
-
Honeydew-associated microbes elicit defense responses against brown planthopper in rice.J Exp Bot. 2019 Mar 11;70(5):1683-1696. doi: 10.1093/jxb/erz041. J Exp Bot. 2019. PMID: 30715410 Free PMC article.
References
-
- Ehrlich P.R., Raven P.H. Butterflies and plants: A study in coevolution. Evolution. 1964;18:586–608.
-
- Labandeira C.C. Insect mouthparts: Ascertaining the paleobiology of insect feeding strategies. Annu. Rev. Ecol. Syst. 1997;28:153–193.
-
- Niklas K.J., Tiffney B.H., Knoll A.H. Patterns in Vascular Land Plant Diversification: An Analysis at the Species Level. In: Valentine J.W., editor. Phanerozoic Diversity Patterns: Profiles in Macroevolution. Princeton University Press; Princeton, NJ, USA: 1985. pp. 97–128.
-
- Schönenberger J., Friis E.M., Matthews M.L., Endress P.K. Cunoniaceae in the cretaceous of europe: Evidence from fossil flowers. Ann. Bot. 2001;88:423–437.
-
- Schaller A., Howe G.A. Direct Defenses in Plants and Their Induction by Wounding and Insect Herbivores. In: Schaller A., editor. Induced Plant Resistance to Herbivory. Springer Science+Business Media; Berlin, Germany: 2008. pp. 7–29.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

