Texas 3-step decellularization protocol: looking at the cardiac extracellular matrix
- PMID: 23681174
- PMCID: PMC3879953
- DOI: 10.1016/j.jprot.2013.05.004
Texas 3-step decellularization protocol: looking at the cardiac extracellular matrix
Abstract
The extracellular matrix (ECM) is a critical tissue component, providing structural support as well as important regulatory signaling cues to govern cellular growth, metabolism, and differentiation. The study of ECM proteins, however, is hampered by the low solubility of ECM components in common solubilizing reagents. ECM proteins are often not detected during proteomics analyses using unbiased approaches due to solubility issues and relatively low abundance compared to highly abundant cytoplasmic and mitochondrial proteins. Decellularization has become a common technique for ECM protein-enrichment and is frequently used in engineering studies. Solubilizing the ECM after decellularization for further proteomic examination has not been previously explored in depth. In this study, we describe testing of a series of protocols that enabled us to develop a novel optimized strategy for the enrichment and solubilization of ECM components. Following tissue decellularization, we use acid extraction and enzymatic deglycosylation to facilitate re-solubilization. The end result is the generation of three fractions for each sample: soluble components, cellular components, and an insoluble ECM fraction. These fractions, developed in mass spectrometry-compatible buffers, are amenable to proteomics analysis. The developed protocol allows identification (by mass spectrometry) and quantification (by mass spectrometry or immunoblotting) of ECM components in tissue samples.
Biological significance: The study of extracellular matrix (ECM) proteins in pathological and non-pathological conditions is often hampered by the low solubility of ECM components in common solubilizing reagents. Additionally, ECM proteins are often not detected during global proteomic analyses due to their relatively low abundance compared to highly abundant cytoplasmic and mitochondrial proteins. In this manuscript we describe testing of a series of protocols that enabled us to develop a final novel optimized strategy for the enrichment and solubilization of ECM components. The end result is the generation of three fractions for each sample: soluble components, cellular components, and an insoluble ECM fraction. By analysis of each independent fraction, differences in protein levels can be detected that in normal conditions would be masked. These fractions are amenable to mass spectrometry analysis to identify and quantify ECM components in tissue samples. The manuscript places a strong emphasis on the immediate practical relevance of the method, particularly when using mass spectrometry approaches; additionally, the optimized method was validated and compared to other methodologies described in the literature.
Copyright © 2013 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
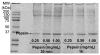
Figures



References
-
- Bosman FT, Stamenkovic I. Functional structure and composition of the extracellular matrix. J Pathol. 2003;200:423–8. http://dx.doi.org/10.1002/path.1437. - DOI - PubMed
-
- Scott-Burden T. Extracellular matrix: the cellular environment. NiPS. 1994;9:110–5.
-
- Didangelos A, Yin X, Mandal K, Baumert M, Jahangiri M, Mayr M. Proteomics characterization of extracellular space components in the human aorta. Mol Cell Proteomics. 2010;9:2048–62. http://dx.doi.org/10.1074/mcp.M110.001693. - DOI - PMC - PubMed
-
- Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech. 2011;4:165–78. http://dx.doi.org/10.1242/dmm.004077. - DOI - PMC - PubMed
-
- Weber KT, Sun Y, Tyagi SC, Cleutjens JP. Collagen network of the myocardium: function, structural remodeling and regulatory mechanisms. J Mol Cell Cardiol. 1994;26:279–92. http://dx.doi.org/10.1006/jmcc.1994.1036. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

