Mechanosensitivity and compositional dynamics of cell-matrix adhesions
- PMID: 23681438
- PMCID: PMC3674437
- DOI: 10.1038/embor.2013.49
Mechanosensitivity and compositional dynamics of cell-matrix adhesions
Abstract
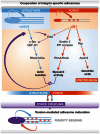
Cells perceive information about the biochemical and biophysical properties of their tissue microenvironment through integrin-mediated cell-matrix adhesions, which connect the cytoskeleton with the extracellular matrix and thereby allow cohesion and long-range mechanical connections within tissues. The formation of cell-matrix adhesions and integrin signalling involves the dynamic recruitment and assembly of an inventory of proteins, collectively termed the 'adhesome', at the adhesive site. The recruitment of some adhesome proteins, most notably the Lin11-, Isl1- and Mec3-domain-containing proteins, depends on mechanical tension generated by myosin II-mediated contractile forces exerted on cell-matrix adhesions. When exposed to force, mechanosensitive adhesome proteins can change their conformation or expose cryptic-binding sites leading to the recruitment of proteins, rearrangement of the cytoskeleton, reinforcement of the adhesive site and signal transduction. Biophysical methods and proteomics revealed force ranges within the adhesome and cytoskeleton, and also force-dependent changes in adhesome composition. In this review, we provide an overview of the compositional dynamics of cell-matrix adhesions, discuss the most prevalent functional domains in adhesome proteins and review literature and concepts about mechanosensing mechanisms that operate at the adhesion site.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

