Efficacy and safety of lisdexamfetamine dimesylate in children with attention-deficit/hyperactivity disorder and recent methylphenidate use
- PMID: 23681505
- PMCID: PMC3680667
- DOI: 10.1007/s12325-013-0027-2
Efficacy and safety of lisdexamfetamine dimesylate in children with attention-deficit/hyperactivity disorder and recent methylphenidate use
Abstract
Introduction: Lisdexamfetamine dimesylate (LDX) is a long-acting prodrug stimulant for the treatment of attention-deficit/hyperactivity disorder (ADHD). Post hoc subgroup analyses were performed from two studies in children with ADHD to compare the efficacy of LDX in participants who had received prior methylphenidate (MPH) treatment with that of the overall study populations.
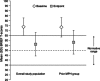
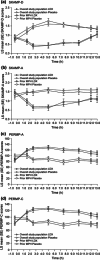
Methods: Study 1 (7-week; open-label design) and study 2 (randomized, double-blind, placebo-controlled, crossover, laboratory school design) enrolled children aged 6-12 years with ADHD and baseline ADHD Rating Scale IV (ADHD-RS-IV) total score ≥28. Both studies excluded children whose prestudy ADHD treatment provided effective control of ADHD symptoms with an acceptable safety profile. Post hoc efficacy analyses were performed in children who had received MPH within 6 months of study enrollment. Efficacy measures included the following scales: ADHD-RS-IV, Clinical Global Impressions-Improvement (CGI-I), Expression and Emotion Scale for Children (EESC), Behavior Rating Inventory of Executive Function (BRIEF), Swanson, Kotkin, Agler, M-Flynn, and Pelham (SKAMP), and Permanent Product Measure of Performance (PERMP).
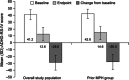
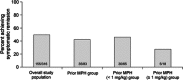
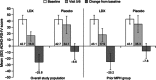
Results: In studies 1 and 2, 83/318 (26%) and 67/129 (52%) participants, respectively, had received MPH within 6 months and were not adequately controlled on current medication with acceptable tolerability; most of these participants had received long-acting MPH. In prior MPH participants, efficacy assessments demonstrated improvements from baseline (study 1) and versus placebo (study 2) that were comparable with those seen in the respective overall study population. Safety profiles were consistent with long-acting stimulant use.
Conclusion: In two studies, children who had received prior MPH treatment improved during treatment with LDX and experienced similar improvements in their symptoms as the overall study populations. For children with ADHD who were previously treated with MPH, LDX may, therefore, be an efficacious treatment option.
Figures

Similar articles
-
Randomized, Double-Blind, Placebo-Controlled Acute Comparator Trials of Lisdexamfetamine and Extended-Release Methylphenidate in Adolescents With Attention-Deficit/Hyperactivity Disorder.CNS Drugs. 2017 Nov;31(11):999-1014. doi: 10.1007/s40263-017-0468-2. CNS Drugs. 2017. PMID: 28980198 Free PMC article.
-
A post hoc comparison of the effects of lisdexamfetamine dimesylate and osmotic-release oral system methylphenidate on symptoms of attention-deficit hyperactivity disorder in children and adolescents.CNS Drugs. 2013 Sep;27(9):743-51. doi: 10.1007/s40263-013-0086-6. CNS Drugs. 2013. PMID: 23801529 Free PMC article. Clinical Trial.
-
The efficacy and safety profile of lisdexamfetamine dimesylate, a prodrug of d-amphetamine, for the treatment of attention-deficit/hyperactivity disorder in children and adults.Clin Ther. 2009 Jan;31(1):142-76. doi: 10.1016/j.clinthera.2009.01.015. Clin Ther. 2009. PMID: 19243715 Review.
-
European, randomized, phase 3 study of lisdexamfetamine dimesylate in children and adolescents with attention-deficit/hyperactivity disorder.Eur Neuropsychopharmacol. 2013 Oct;23(10):1208-18. doi: 10.1016/j.euroneuro.2012.11.012. Epub 2013 Jan 15. Eur Neuropsychopharmacol. 2013. PMID: 23332456 Clinical Trial.
-
Systematic evidence synthesis of treatments for ADHD in children and adolescents: indirect treatment comparisons of lisdexamfetamine with methylphenidate and atomoxetine.Curr Med Res Opin. 2014 Aug;30(8):1673-85. doi: 10.1185/03007995.2014.904772. Epub 2014 Apr 15. Curr Med Res Opin. 2014. PMID: 24627974
Cited by
-
Comparative efficacy, acceptability, and tolerability of lisdexamfetamine in child and adolescent ADHD: a meta-analysis of randomized, controlled trials.Drug Des Devel Ther. 2015 Apr 1;9:1927-36. doi: 10.2147/DDDT.S79071. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25897203 Free PMC article. Review.
-
Update on optimal use of lisdexamfetamine in the treatment of ADHD.Neuropsychiatr Dis Treat. 2013;9:977-83. doi: 10.2147/NDT.S34092. Epub 2013 Jul 22. Neuropsychiatr Dis Treat. 2013. PMID: 23901276 Free PMC article.
-
New Drugs to Treat ADHD: Opportunities and Challenges in Research and Development.Curr Top Behav Neurosci. 2022;57:79-126. doi: 10.1007/7854_2022_332. Curr Top Behav Neurosci. 2022. PMID: 35507283 Review.
-
Lisdexamfetamine dimesylate: a new therapeutic option for attention-deficit hyperactivity disorder.CNS Drugs. 2012 Aug 1;26(8):691-705. doi: 10.2165/11634340-000000000-00000. CNS Drugs. 2012. PMID: 22762726 Review.
-
New Formulations of Stimulants: An Update for Clinicians.J Child Adolesc Psychopharmacol. 2019 Jun;29(5):324-339. doi: 10.1089/cap.2019.0043. Epub 2019 Apr 30. J Child Adolesc Psychopharmacol. 2019. PMID: 31038360 Free PMC article. Review.
References
-
- Rader R, McCauley L, Callen EC. Current strategies in the diagnosis and treatment of childhood attention-deficit/hyperactivity disorder. Am Fam Physician. 2009;79:657–665. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

