Usp18 deficient mammary epithelial cells create an antitumour environment driven by hypersensitivity to IFN-λ and elevated secretion of Cxcl10
- PMID: 23681607
- PMCID: PMC3721472
- DOI: 10.1002/emmm.201201864
Usp18 deficient mammary epithelial cells create an antitumour environment driven by hypersensitivity to IFN-λ and elevated secretion of Cxcl10
Abstract
The theory of cancer immunoediting refers to mechanisms by which the immune system can suppress or promote tumour progression. A major challenge for the development of novel cancer immunotherapies is to find ways to exploit the immune system's antitumour activity while concomitantly reducing its protumour activity. Using the PyVmT model of mammary tumourigenesis, we show that lack of the Usp18 gene significantly inhibits tumour growth by creating a tumour-suppressive microenvironment. Generation of this antitumour environment is driven by elevated secretion of the potent T-cell chemoattractant Cxcl10 by Usp18 deficient mammary epithelial cells (MECs), which leads to recruitment of Th1 subtype CD4(+) T cells. Furthermore, we show that Cxcl10 upregulation in MECs is promoted by interferon-λ and that Usp18 is a novel inhibitor of interferon-λ signalling. Knockdown of the interferon-λ specific receptor subunit IL-28R1 in Usp18 deficient MECs dramatically enhances tumour growth. Taken together, our data suggest that targeting Usp18 may be a viable approach to boost antitumour immunity while suppressing the protumour activity of the immune system.
Keywords: Cxcl10; UBP43; Usp18; breast cancer; interferon-λ.
© 2013 The Authors. Published by John Wiley and Sons, Ltd on behalf of EMBO.
Figures

Percentage of tumour-free mice shown by Kaplan–Meier plot. PyVmT/Usp18 WT and KO mice were palpated twice a week for mammary tumours. n = 10 for each cohort.
Kaplan–Meier curves for survival of PyVmT/Usp18 WT and KO mice. A mean tumour diameter of 0.5 cm was used as endpoint for the survival studies. PyVmT/Usp18 WT, n = 5; PyVmT/Usp18 KO, n = 3.
Representative photograph of a PyVmT/Usp18 WT and PyVmT/Usp18 KO mouse at 13 weeks of age.
PyVmT mice were sacrificed at 13 weeks of age and tumour burden (tumour weight/body weight) determined. PyVmT/Usp18 WT, n = 20; PyVmT/Usp18 KO, n = 20.
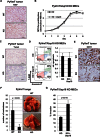
Paraffin-embedded tumour tissues were analyzed for proliferation marker Ki67 by immunohistochemistry. Images are 200× with 200 µm scale bar.
Proliferative capacity of transduced primary tumour cells was analyzed in vitro by MTS assay.
Number of apoptotic cells was determined with TUNEL assay on paraffin-embedded tumour tissues. Images are 200× with 200 µm scale bar.
Percentage of apoptotic cells in transduced primary tumour cells was analyzed by AnnexinV staining (left panel) and relative apoptosis from three independent experiments determined (right panel).
Immunohistochemical analysis of frozen tumour sections for angiogenesis marker CD31. Images are 200× with 200 µm scale bar.
Number of spontaneous lung metastases in PyVmT mice of 13 weeks of age was determined by serial lung sections stained with H&E. N = 5 mice per group. Values shown represent mean total number of lung metastases ± SD (left panel). Representative photographs of lungs excised from PyVmT/Usp18 WT or PyVmT/Usp18 KO mice are shown (right panel). Macroscopically visible surface metastases are marked with “M.”
Invasive potential of PyVmT/Usp18 KO MECs was determined in an in vitro assay using invasion chambers coated with matrigel. Shown are combined results of three independent experiments.
If statistical significance was reached relevant p values are shown in the diagram.
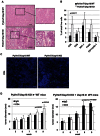
H&E staining of paraffin-embedded mammary tumour tissue. Images are 100× with 500 µm scale bar.
Single cell suspensions from tumours of PyVmT/Usp18 WT and PyVmT/Usp18 KO mice were analyzed for immune cell infiltration by flow cytometry. WT, n = 7; KO, n = 7.
Frozen sections of mammary tumours stained for CD4+cells were analyzed by immunofluorescence. Images are 200× with 100 µm scale bar.
Mice treated with either anti-CD4+antibody or control IgG were injected with PyVmT/Usp18 KO MECs or PyVmT/Usp18 KO + Usp18, respectively. Tumour growth was monitored weekly by measuring tumours with a calliper. Number of tumours analyzed: CD4, n = 6; IgG, n = 6.
If statistical significance was reached relevant p values are shown in the diagram.

Transcript levels of Cxcr3 ligands and IFN genes Irf7 and Oas2 were analyzed by qRT-PCR in both tumours and transduced PyVmT/Usp18 KO MECs. The relative means from two separate tumours per genotype normalized to WT (left panel) or relative means from three independent experiments normalized to Usp18 (right panel) are shown.
Cxcl10 protein levels in the culture medium supernatant of transduced PyVmT/Usp18 KO MECs were determined by ELISA. Left panel shows representative experiment with total values and right panel shows relative Cxcl10 levels from three independent experiments.
Cytokines in the tumour microenvironment associated with T-cell subtypes were analyzed by qRT-PCR. Mean values of two separate tumours per genotype normalized to WT are shown. Experiment was performed in triplicate.
Cytokines in the tumour microenvironment associated with macrophage subtypes were analyzed by qRT-PCR. Mean values of two separate tumours per genotype normalized to WT are shown. Experiment was performed in triplicate.
If statistical significance was reached relevant p values are shown in the diagram.
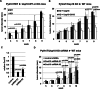
Wild-type PyVmT/MECs were injected into two mammary fat pads per Usp18 KO or WT mouse. Tumour growth was monitored weekly by measuring tumours with a calliper. Number of tumours analyzed: WT, n = 8; KO, n = 7.
Control vector (KO), Usp18 (KO + Usp18) or Usp18-C61S (KO + Usp18-C61S) transduced PyVmT/Usp18 KO MECs were injected into two mammary fat pads per WT mouse. Tumour growth was monitored weekly by measuring tumours with a calliper. Number of tumours analyzed: KO, n = 8; KO + Usp18, n = 8; KO + Usp18-C61S, n = 7.
Cxcl10 protein levels in the culture medium supernatant of Cxcl10 knockdown PyVmT/Usp18 KO MECs was determined by ELISA. Experiment was performed in triplicate.
PyVmT/Usp18 KO MECs transduced with control or Cxcl10 shRNAs were injected into two mammary fat pads per wild-type mouse. Tumour growth was monitored weekly by measuring tumours with a calliper. Number of tumours analyzed: Control shRNA n = 10, Cxcl10 shRNA#1 n = 10, Cxcl10 shRNA#2 n = 9.
If statistical significance was reached relevant p values are shown in the diagram.

Induction of Cxcr3 ligands, Irf7 and Oas2 upon IFN-β or IFN-λ treatment in parental MECs was analyzed by qRT-PCR. Relative means from three independent experiments normalized to IFN-β treated cells are shown.
Induction of Cxcr3 ligands, Irf7 and Oas2 upon IFN-β or IFN-λ treatment in transduced PyVmT/Usp18 KO MECs was analyzed by qRT-PCR. Relative means from three independent experiments normalized to untreated cells are shown.
Phosphorylation of StatT1 in control vector (KO) or Usp18 (KO + Usp18) transduced PyVmT/Usp18 KO MECs upon IFN-β or IFN-λ treatment was determined by Western blotting. Cells were left untreated or IFN treated for 15 min before analysis. Cells were harvested, lysed and analyzed for Usp18, Stat1 and phosphorylated Stat1 levels by Western blotting. Tubulin was used as loading control.
Tumour lysates from PyVmT/WT and PyVmT/Usp18 KO mice were analysed for p-Stat1, total Stat1 and Cxcl10 by Western blotting. Tubulin was used as loading control.
Knockdown efficiency of PyVmT/Usp18 KO MECs stably expressing IL-28R1 shRNA was analyzed by IL-28R1 Western blotting. Tubulin was used as loading control.
PyVmT/Usp18 KO MECs transduced with control or IL-28R1 shRNA were injected into mammary fat pads of wild-type mice. Tumour growth was monitored weekly by measuring tumours with a calliper. Number of tumours analyzed: Control shRNA, n = 5; IL-28R1 shRNA, n = 5.
If statistical significance was reached relevant p values are shown in the diagram.
References
-
- Bidwell BN, Slaney CY, Withana NP, Forster S, Cao Y, Loi S, Andrews D, Mikeska T, Mangan NE, Samarajiwa SA, et al. Silencing of Irf7 pathways in breast cancer cells promotes bone metastasis through immune escape. Nat Med. 2012;18:1224–1231. - PubMed
-
- Coccia EM, Severa M, Giacomini E, Monneron D, Remoli ME, Julkunen I, Cella M, Lande R, Uze G. Viral infection and Toll-like receptor agonists induce a differential expression of type I and lambda interferons in human plasmacytoid and monocyte-derived dendritic cells. Eur J Immunol. 2004;34:796–805. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

