Organelle asymmetry for proper fitness, function, and fate
- PMID: 23681659
- PMCID: PMC4121668
- DOI: 10.1007/s10577-013-9350-3
Organelle asymmetry for proper fitness, function, and fate
Abstract
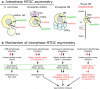
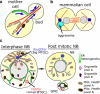
During cellular division, centrosomes are tasked with building the bipolar mitotic spindle, which partitions the cellular contents into two daughter cells. While every cell will receive an equal complement of chromosomes, not every organelle is symmetrically passaged to the two progeny in many cell types. In this review, we highlight the conservation of nonrandom centrosome segregation in asymmetrically dividing stem cells, and we discuss how the asymmetric function of centrosomes could mediate nonrandom segregation of organelles and mRNA. We propose that such a mechanism is critical for insuring proper cell fitness, function, and fate.
Figures


References
-
-
Alliegro MC, Alliegro MA, Palazzo RE. Centrosome-associated RNA in surf clam oocytes. Proc Natl Acad Sci U S A. 2006;103(24):9034–9038. [Research Support, N.I.H., Extramural].
-
-
-
Anderson CT, Stearns T. Centriole age underlies asynchronous primary cilium growth in mammalian cells. Curr Biol. 2009;19(17):1498–1502. [Research Support, N.I.H., Extramural. Research Support, U.S. Gov't, Non-P.H.S.].
-
-
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449(7165):1003–1007. - PubMed
-
-
Bellaiche Y, Beaudoin-Massiani O, Stuttem I, Schweisguth F. The planar cell polarity protein Strabismus promotes Pins anterior localization during asymmetric division of sensory organ precursor cells in Drosophila. Development. 2004;131(2):469–478. [Research Support, Non-U.S. Gov't].
-
-
-
Berger C, Harzer H, Burkard TR, Steinmann J, van der Horst S, Laurenson AS, et al. FACS purification and transcriptome analysis of Drosophila neural stem cells reveals a role for Klumpfuss in self-renewal. Cell Reports. 2012;2(2):407–418. [Research Support, Non-U.S. Gov't].
-
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

