A TSPO ligand is protective in a mouse model of multiple sclerosis
- PMID: 23681668
- PMCID: PMC3779450
- DOI: 10.1002/emmm.201202124
A TSPO ligand is protective in a mouse model of multiple sclerosis
Abstract
Local production of neurosteroids such as progesterone and allopregnanolone confers neuroprotection in central nervous system (CNS) inflammatory diseases. The mitochondrial translocator protein (TSPO) performs a rate-limiting step in the conversion of cholesterol to pregnenolone and its steroid derivatives. Previous studies have shown that TSPO is upregulated in microglia and astroglia during neural inflammation, and radiolabelled TSPO ligands such as PK11195 have been used to image and localize injury in the CNS. Recent studies have shown that modulating TSPO activity with pharmacological ligands such as etifoxine can initiate the production of neurosteroids locally in the injured CNS. In this study, we examined the effects of etifoxine, a clinically available anxiolytic drug, in the development and progression of mouse experimental autoimmune encephalomyelitis (EAE), an experimental model for multiple sclerosis (MS). Our results showed that etifoxine attenuated EAE severity when administered before the development of clinical signs and also improved symptomatic recovery when administered at the peak of the disease. In both cases, recovery was correlated with diminished inflammatory pathology in the lumbar spinal cord. Modulation of TSPO activity by etifoxine led to less peripheral immune cell infiltration of the spinal cord, and increased oligodendroglial regeneration after inflammatory demyelination in EAE. Our results suggest that a TSPO ligand, e.g. etifoxine, could be a potential new therapeutic option for MS with benefits that could be comparable to the administration of systemic steroids but potentially avoiding the detrimental side effects of long-term direct use of steroids.
Copyright © 2013 The Authors. Published by John Wiley and Sons, Ltd on behalf of EMBO.
Figures

TSPO was generally expressed at low levels in the normal adult spinal cord.
In contrast, TSPO was markedly upregulated in EAE mice.
The main site of TSPO in healthy mice was the central canal ependymal cells, which remained in similar high levels of TSPO expression after EAE.
TSPO was also expressed in a small population of NG2+ cells in the normal state and remained similar in the disease state.
TSPO was observed in activated astrocytes (GFAP+) in EAE mice.
The majority of the cells expressing TSPO were activated microglia and infiltrating macrophages (CD68+) in EAE mice.
TSPO mRNA levels also showed a dramatic increase in response to inflammatory signals. EAE spinal cords (*p = 0.004), as well as astrocytes (*p = 0.006) and microglia (*p = 0.01) treated with IFN-γ all showed an increase in TSPO mRNA levels. The p-values were calculated by t-test, n = 8/group. Scale bar = 50 µm.
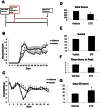
A. Etifoxine treatment was started on day 7 post induction, and was administered daily i.p. at a dose of 50 mg/kg.
B. The drug treated group showed a decrease in clinical scoring at days 14–16.
C. The drug treated group showed a significant increase in weight retention during the peak of clinical symptoms.
D–G. The mice showed a significant decrease in the total scoring (*p = 0.000001) (D), an increase in the survival rate (E), a decrease in the maximal score (*p = 0.03) (F), and a delayed onset of clinical scoring (*p = 0.04) (G). The p-values for EAE clinical scores and weight were calculated using Mann–Whitney test, for total scores using Wilcoxon signed-rank test, and for days till onset and maximal score using t-test, n = 10 mice/group.
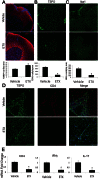
A–D. At day 10 p.i., drug treated animals showed significant differences in MBP (A), TSPO (B), Iba1 (C) and CD4 staining (D). (A) Animals treated with etifoxine showed an increase in retention of percentage of MBP coverage (*p = 0.001). Etifoxine treated animals also exhibited a decrease in TSPO+ cells per mm2 (*p = 0.004) (B), along with less Iba1+ cells per mm2 (*p = 0.02) (C). (D) The vehicle group showed infiltration of CD4+ cells, while no CD4+ cells were seen in the drug treated group. Infiltrated CD4+ cells were positive for TSPO expression.
E. The mRNA levels corroborated the CD4 histology. Along with the decrease in CD4 mRNA (*p = 0.01), there was a decrease in transcripts of the inflammatory cytokines interferon-γ (IFN-γ) (*p = 0.02) and interleukin 17 (IL-17) (*p = 0.04) in animals treated with etifoxine. The p-values were calculated by t-test, n = 8/group.
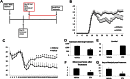
A–C. Treatment of etifoxine was started at the peak of EAE (day 18), and continued daily at a dose of 50 mg/kg i.p. (A) The drug treated group showed a decrease in clinical scores (B), with an increase in weight gain (C).
D–G. The etifoxine group also showed a decrease in the total scoring (D), greater animal survival (E), a decrease in the mean low score (*p = 0.01) (F), and a decrease in days of paralysis after treatment (*p = 0.004) (G). The p-values for EAE clinical scores and weight were calculated using Mann–Whitney test, for total scores using Wilcoxon signed-rank test, and for the mean low score and days of paralysis after treatment using t-test, n = 12 mice/group.

Cells were then measured as a percentage of the total cell population. There was a decrease in the percentage of CD4+ (*p = 0.04), CD4+/IL-17+ (*p = 0.04), CD8+ (*p = 0.01) and CD8+/IFNγ+ (*p = 0.03) cells in the drug-treated group
Protein levels of the inflammatory cytokines IL-1β (*p = 0.001), IL-17 (*p = 0.02) and IFN-γ (*p = 0.02) were also measured, and there was a significant decrease in all cytokines in response to etifoxine treatment
The p-values were calculated using t-test, n = 8/group.

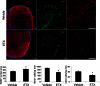
There was a significant increase in the NG2+ cell population in the etifoxine treated group (*p = 0.01) compared to the vehicle treated group, and there was also an increase in Olig2+ cells.
NG2+ cells were found on the dorsal side of the central canal, and extended toward sites of injury, and co-labelled with TSPO.
The mRNA levels of Plp (*p = 0.007) and Olig2 (*p = 0.02) were increased in the drug treated group, so were the levels of akr1c1 (*p = 0.03), the main 3α-hydroxysteroid dehydrogenase (3αHSD) that is responsible for the biosynthesis of the neurosteroid allopregnanolone. The p-values were calculated using t-test, n = 8/group. Scale bar = 50 µm.

Low magnification electron micrograph showed a large number of degenerating myelinated axons (dAx) and microglia/macrophage cells (M) in the spinal cord white matter of the vehicle-treated EAE mice. Note that the degenerating axons displayed various morphological features, but they all showed disorganized myelin sheaths and electron-dense or swollen axoplasm. Microglia/macrophage cells (M) were frequently found to surround or be close to the degenerating axons. Scale bar = 5 µm.
High magnification electron micrograph taken from the same section in panel A, demonstrating that several microglia cells surrounding a degenerating axon (dAx) where the myelin sheath completely collapsed and the axoplasm became transparent, and indicating few organelles existed at this advanced stage of degeneration. Another degenerating axon (dAx) was seen near the microglia/macrophage (M). Scale bar = 1 µm.
Low magnification electron micrograph showing the spinal cord white matter of etifoxine-treated mice after EAE. Most of the axons (Ax) were myelinated and axonal organelles such as mitochrondria were present. No microglia or macrophages were found associating with myelinated axons. Scale bar = 5 µm.
High magnification electron micrograph showing myelinated axons (Ax), while mitochrondria appearing normal and neurofilaments being well preserved in the axoplasm. Inset showing higher magnification image of a myelinated axon (asterisk), with myelin sheath layers being clearly identified, and the major dense lines and the intraperiod lines also being clearly seen. Scale bar in D = 1 µm and in the inset = 0.1 µm.
Similar articles
-
Differential efficacy of the TSPO ligands etifoxine and XBD-173 in two rodent models of Multiple Sclerosis.Neuropharmacology. 2016 Sep;108:229-37. doi: 10.1016/j.neuropharm.2016.03.053. Epub 2016 Mar 30. Neuropharmacology. 2016. PMID: 27039042
-
A TSPO ligand attenuates brain injury after intracerebral hemorrhage.FASEB J. 2017 Aug;31(8):3278-3287. doi: 10.1096/fj.201601377RR. Epub 2017 Apr 17. FASEB J. 2017. PMID: 28416580 Free PMC article.
-
A translocator protein 18 kDa agonist protects against cerebral ischemia/reperfusion injury.J Neuroinflammation. 2017 Jul 28;14(1):151. doi: 10.1186/s12974-017-0921-7. J Neuroinflammation. 2017. PMID: 28754131 Free PMC article.
-
Neurosteroids and translocator protein 18 kDa (TSPO) in depression: implications for synaptic plasticity, cognition, and treatment options.Eur Arch Psychiatry Clin Neurosci. 2023 Oct;273(7):1477-1487. doi: 10.1007/s00406-022-01532-3. Epub 2022 Dec 27. Eur Arch Psychiatry Clin Neurosci. 2023. PMID: 36574032 Review.
-
Translocator protein ligands as promising therapeutic tools for anxiety disorders.Curr Med Chem. 2009;16(26):3359-80. doi: 10.2174/092986709789057653. Epub 2009 Sep 1. Curr Med Chem. 2009. PMID: 19548867 Review.
Cited by
-
Impact of Intraventricular Human Adipose-Derived Stem Cells Transplantation with Pregnenolone Treatment on Remyelination of Corpus Callosum in A Rat Model of Multiple Sclerosis.Cell J. 2022 Dec 1;24(12):748-756. doi: 10.22074/cellj.2022.8173. Cell J. 2022. PMID: 36527347 Free PMC article.
-
The role of glial cells in multiple sclerosis disease progression.Nat Rev Neurol. 2022 Apr;18(4):237-248. doi: 10.1038/s41582-022-00624-x. Epub 2022 Feb 21. Nat Rev Neurol. 2022. PMID: 35190704 Review.
-
Translocator Protein Ligand Protects against Neurodegeneration in the MPTP Mouse Model of Parkinsonism.J Neurosci. 2019 May 8;39(19):3752-3769. doi: 10.1523/JNEUROSCI.2070-18.2019. Epub 2019 Feb 22. J Neurosci. 2019. PMID: 30796158 Free PMC article.
-
Translocator Protein Ligand PIGA1138 Reduces Disease Symptoms and Severity in Experimental Autoimmune Encephalomyelitis Model of Primary Progressive Multiple Sclerosis.Mol Neurobiol. 2022 Mar;59(3):1744-1765. doi: 10.1007/s12035-022-02737-2. Epub 2022 Jan 11. Mol Neurobiol. 2022. PMID: 35018577
-
VDAC1 and the TSPO: Expression, Interactions, and Associated Functions in Health and Disease States.Int J Mol Sci. 2019 Jul 8;20(13):3348. doi: 10.3390/ijms20133348. Int J Mol Sci. 2019. PMID: 31288390 Free PMC article. Review.
References
-
- Cagnin A, Rossor M, Sampson EL, Mackinnon T, Banati RB. In vivo detection of microglial activation in frontotemporal dementia. Ann Neurol. 2004;56:894–897. - PubMed
-
- Choi HB, Khoo C, Ryu JK, van Breemen E, Kim SU, McLarnon JG. Inhibition of lipopolysaccharide-induced cyclooxygenase-2, tumor necrosis factor-α and [Ca2+]i responses in human microglia by the peripheral benzodiazepine receptor ligand PK11195. J Neurochem. 2002;83:546–555. - PubMed
-
- Edison P, Archer HA, Gerhard A, Hinz R, Pavese N, Turkheimer FE, Hammers A, Tai YF, Fox N, Kennedy A, et al. Microglia, amyloid, and cognition in Alzheimer's disease: an 11C(R)PK11195-PET and [11C]PIB-PET study. Neurobiol Dis. 2008;32:412–419. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

