Inhaled, dual release liposomal ciprofloxacin in non-cystic fibrosis bronchiectasis (ORBIT-2): a randomised, double-blind, placebo-controlled trial
- PMID: 23681906
- PMCID: PMC4770250
- DOI: 10.1136/thoraxjnl-2013-203207
Inhaled, dual release liposomal ciprofloxacin in non-cystic fibrosis bronchiectasis (ORBIT-2): a randomised, double-blind, placebo-controlled trial
Abstract
Background: The delivery of antipseudomonal antibiotics by inhalation to Pseudomonas aeruginosa-infected subjects with non-cystic fibrosis (CF) bronchiectasis is a logical extension of treatment strategies successfully developed in CF bronchiectasis. Dual release ciprofloxacin for inhalation (DRCFI) contains liposomal ciprofloxacin, formulated to optimise airway antibiotic delivery.
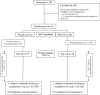
Methods: Phase II, 24-week Australian/New Zealand multicentre, randomised, double-blind, placebo-controlled trial in 42 adult bronchiectasis subjects with ≥2 pulmonary exacerbations in the prior 12 months and ciprofloxacin-sensitive P aeruginosa at screening. Subjects received DRCFI or placebo in three treatment cycles of 28 days on/28 days off. The primary outcome was change in sputum P aeruginosa bacterial density to the end of treatment cycle 1 (day 28), analysed by modified intention to treat (mITT). Key secondary outcomes included safety and time to first pulmonary exacerbation-after reaching the pulmonary exacerbation endpoint subjects discontinued study drug although remained in the study.
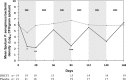
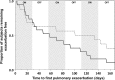
Results: DRCFI resulted in a mean (SD) 4.2 (3.7) log10 CFU/g reduction in P aeruginosa bacterial density at day 28 (vs -0.08 (3.8) with placebo, p=0.002). DRCFI treatment delayed time to first pulmonary exacerbation (median 134 vs 58 days, p=0.057 mITT, p=0.046 per protocol). DRCFI was well tolerated with a similar incidence of systemic adverse events to the placebo group, but fewer pulmonary adverse events.
Conclusions: Once-daily inhaled DRCFI demonstrated potent antipseudomonal microbiological efficacy in adults with non-CF bronchiectasis and ciprofloxacin-sensitive P aeruginosa. In this modest-sized phase II study, DRCFI was also well tolerated and delayed time to first pulmonary exacerbation in the per protocol population.
Keywords: Bronchiectasis; Respiratory Infection.
Figures
Similar articles
-
Inhaled liposomal ciprofloxacin in patients with non-cystic fibrosis bronchiectasis and chronic lung infection with Pseudomonas aeruginosa (ORBIT-3 and ORBIT-4): two phase 3, randomised controlled trials.Lancet Respir Med. 2019 Mar;7(3):213-226. doi: 10.1016/S2213-2600(18)30427-2. Epub 2019 Jan 15. Lancet Respir Med. 2019. PMID: 30658914 Clinical Trial.
-
Microbiological changes observed over 48 weeks of treatment with inhaled liposomal ciprofloxacin in individuals with non-cystic fibrosis bronchiectasis and chronic Pseudomonas aeruginosa lung infection.Clin Microbiol Infect. 2019 Dec;25(12):1532-1538. doi: 10.1016/j.cmi.2019.04.017. Epub 2019 Apr 26. Clin Microbiol Infect. 2019. PMID: 31035017 Clinical Trial.
-
Addition of inhaled tobramycin to ciprofloxacin for acute exacerbations of Pseudomonas aeruginosa infection in adult bronchiectasis.Chest. 2006 Nov;130(5):1503-10. doi: 10.1378/chest.130.5.1503. Chest. 2006. PMID: 17099030 Clinical Trial.
-
Spotlight on inhaled ciprofloxacin and its potential in the treatment of non-cystic fibrosis bronchiectasis.Drug Des Devel Ther. 2018 Nov 27;12:4059-4066. doi: 10.2147/DDDT.S168014. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 30568427 Free PMC article. Review.
-
Efficacy and safety of long-term inhaled antibiotic for patients with noncystic fibrosis bronchiectasis: a meta-analysis.Clin Respir J. 2016 Nov;10(6):731-739. doi: 10.1111/crj.12278. Epub 2015 Mar 2. Clin Respir J. 2016. PMID: 25620629 Review.
Cited by
-
Tuning Ciprofloxacin Release Profiles from Liposomally Encapsulated Nanocrystalline Drug.Pharm Res. 2016 Nov;33(11):2748-62. doi: 10.1007/s11095-016-2002-5. Epub 2016 Jul 20. Pharm Res. 2016. PMID: 27439506 Free PMC article.
-
Evidence of inhaled tobramycin in non-cystic fibrosis bronchiectasis.Open Respir Med J. 2015 Mar 31;9:30-6. doi: 10.2174/1874306401509010030. eCollection 2015. Open Respir Med J. 2015. PMID: 25893022 Free PMC article.
-
Biomaterial therapeutic strategies for treatment of bacterial lung infections.Biofilm. 2023 Mar 1;5:100111. doi: 10.1016/j.bioflm.2023.100111. eCollection 2023 Dec. Biofilm. 2023. PMID: 36909663 Free PMC article.
-
Psychometric Validation of the German Translation of the Quality of Life Questionnaire-Bronchiectasis (QOL-B)-Data from the German Bronchiectasis Registry PROGNOSIS.J Clin Med. 2022 Jan 15;11(2):441. doi: 10.3390/jcm11020441. J Clin Med. 2022. PMID: 35054135 Free PMC article.
-
Evaluating hemoptysis hospitalizations among patients with bronchiectasis in the United States: a population-based cohort study.BMC Pulm Med. 2021 Dec 1;21(1):392. doi: 10.1186/s12890-021-01762-6. BMC Pulm Med. 2021. PMID: 34852812 Free PMC article.
References
-
- Wilson CB, Jones PW, O'Leary CJ, et al. Effect of sputum bacteriology on the quality of life of patients with bronchiectasis. Eur Respir J 1997;10:1754–60. - PubMed
-
- King PT, Holdsworth SR, Freezer NJ, et al. Microbiologic follow-up study in adult bronchiectasis. Respir Med 2007;101:1633–8. - PubMed
-
- Martinez-Garcia MA, Soler-Cataluna JJ, Perpina-Tordera M, et al. Factors associated with lung function decline in adult patients with stable non-cystic fibrosis bronchiectasis. Chest 2007;132:1565–72. - PubMed
-
- Ramsey BW, Pepe MS, Quan JM, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. N Engl J Med 1999;340:23–30. - PubMed
-
- O'Donnell AE, Barker AF, Ilowite JS, et al. Treatment of idiopathic bronchiectasis with aerosolized recombinant human DNase 1. Chest 1998;113:1329–34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
