Highly efficient differentiation of functional hepatocytes from human induced pluripotent stem cells
- PMID: 23681950
- PMCID: PMC3673753
- DOI: 10.5966/sctm.2012-0160
Highly efficient differentiation of functional hepatocytes from human induced pluripotent stem cells
Abstract
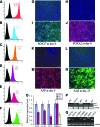
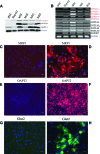
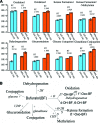
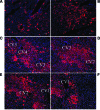
Human induced pluripotent stem cells (hiPSCs) hold great potential for use in regenerative medicine, novel drug development, and disease progression/developmental studies. Here, we report highly efficient differentiation of hiPSCs toward a relatively homogeneous population of functional hepatocytes. hiPSC-derived hepatocytes (hiHs) not only showed a high expression of hepatocyte-specific proteins and liver-specific functions, but they also developed a functional biotransformation system including phase I and II metabolizing enzymes and phase III transporters. Nuclear receptors, which are critical for regulating the expression of metabolizing enzymes, were also expressed in hiHs. hiHs also responded to different compounds/inducers of cytochrome P450 as mature hepatocytes do. To follow up on this observation, we analyzed the drug metabolizing capacity of hiHs in real time using a novel ultra performance liquid chromatography-tandem mass spectrometry. We found that, like freshly isolated primary human hepatocytes, the seven major metabolic pathways of the drug bufuralol were found in hiHs. In addition, transplanted hiHs engrafted, integrated, and proliferated in livers of an immune-deficient mouse model, and secreted human albumin, indicating that hiHs also function in vivo. In conclusion, we have generated a method for the efficient generation of hepatocytes from induced pluripotent stem cells in vitro and in vivo, and it appears that the cells function similarly to primary human hepatocytes, including developing a complete metabolic function. These results represent a significant step toward using patient/disease-specific hepatocytes for cell-based therapeutics as well as for pharmacology and toxicology studies.
Keywords: Hepatocyte differentiation; Induced pluripotent stem cells; Liver regeneration; Stem cell transplantation.
Figures



References
-
- Cai J, Zhao Y, Liu Y, et al. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007;45:1229–1239. - PubMed
-
- Hay DC, Zhao D, Fletcher J, et al. Efficient differentiation of hepatocytes from human embryonic stem cells exhibiting markers recapitulating liver development in vivo. Stem Cells. 2008;26:894–902. - PubMed
-
- Agarwal S, Holton KH, Lanza R. Efficient differentiation of functional hepatocytes from human embryonic stem cells. Stem Cells. 2008;26:1117–1127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

