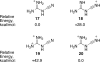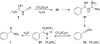Benzamide synthesis by direct electrophilic aromatic substitution with cyanoguanidine
- PMID: 23682199
- PMCID: PMC3652529
- DOI: 10.1016/j.tetlet.2012.06.135
Benzamide synthesis by direct electrophilic aromatic substitution with cyanoguanidine
Abstract
Cyanoguanidine is an inexpensive commodity chemical and it is found to be a useful reagent for the direct Friedel-Crafts carboxamidation of arenes. The reaction works best in an excess of Brønsted superacid, an observation suggesting the involvement of a superelectrophilic intermediate. Theoretical calculations indicate that the most stable diprotonated species involves protonation at the guanidine and cyano nitrogen atoms.
Keywords: Friedel-Crafts; benzamide; carboxamidation; superacid; superelectrophile.
Figures
References
-
- Wu X-F, Neumann H, Beller M. Chem. Asian J. 2010;5:2168–2172. - PubMed
-
and references cited therein.
-
- Gauvreau D, Dolman SJ, Hughes G, O'Shea PD, Davies IW. J. Org. Chem. 2010;75:4078. - PubMed
- Effenberger F, Gleiter R. Chem. Ber. 1964;97:472.
-
- Kozyukov VP, Kozyukov Vik. P., Muzovskaya EV, Mironov VF. Zhur. Obs. Khim. 1989;59:1202–1203.
- Graf R. German Pat. DE 1010958 (1957)
Grants and funding
LinkOut - more resources
Full Text Sources