Chemotherapy for prostate cancer: Clinical practice in Canada
- PMID: 23682304
- PMCID: PMC3652211
- DOI: 10.5489/cuaj.273
Chemotherapy for prostate cancer: Clinical practice in Canada
Abstract
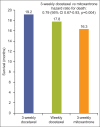
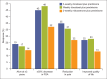
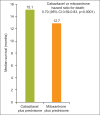
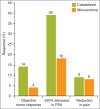
Whereas prostate cancer was once deemed unresponsive to chemotherapy, there is now evidence that patients with metastatic castration-resistant prostate cancer can obtain a survival benefit from both first-line (docetaxel-based) and second-line (cabazitaxel-based) chemotherapy. The side effects of these agents have been shown to be predictable and manageable, particularly in North American centres. However, patient selection remains a key issue, with the aim of delivering each line of treatment at a time when the individual patient remains fit and well enough to tolerate a cytotoxic regimen. Hence, it is increasingly important for urologists and oncologists to work together to ensure timely consideration of the chemotherapeutic approach before it is precluded by a decline in performance status.
Figures


Similar articles
-
Custirsen (OGX-011) combined with cabazitaxel and prednisone versus cabazitaxel and prednisone alone in patients with metastatic castration-resistant prostate cancer previously treated with docetaxel (AFFINITY): a randomised, open-label, international, phase 3 trial.Lancet Oncol. 2017 Nov;18(11):1532-1542. doi: 10.1016/S1470-2045(17)30605-8. Epub 2017 Oct 9. Lancet Oncol. 2017. PMID: 29033099 Clinical Trial.
-
Practical guide to the use of chemotherapy in castration resistant prostate cancer.Can J Urol. 2014 Apr;21(2 Supp 1):77-83. Can J Urol. 2014. PMID: 24775728 Review.
-
Optimizing the care of patients with advanced prostate cancer in the UK: current challenges and future opportunities.BJU Int. 2012 Sep;110(5):658-67. doi: 10.1111/j.1464-410X.2011.10886.x. Epub 2012 Mar 19. BJU Int. 2012. PMID: 22429837
-
Treating Patients with Metastatic Castration Resistant Prostate Cancer: A Comprehensive Review of Available Therapies.J Urol. 2015 Dec;194(6):1537-47. doi: 10.1016/j.juro.2015.06.106. Epub 2015 Jul 18. J Urol. 2015. PMID: 26196735 Review.
-
An observational, multicentre study of cabazitaxel in patients with metastatic castration-resistant prostate cancer previously treated with docetaxel (CAPRISTANA).BJU Int. 2019 Mar;123(3):456-464. doi: 10.1111/bju.14509. Epub 2018 Sep 16. BJU Int. 2019. PMID: 30098093 Free PMC article.
Cited by
-
Post-docetaxel options for further survival benefit in metastatic castration-resistant prostate cancer: Questions of choice.Can Urol Assoc J. 2013 Jan-Feb;7(1-2 Suppl 1):S11-7. doi: 10.5489/cuaj.274. Can Urol Assoc J. 2013. PMID: 23682301 Free PMC article.
-
Optimal management of patients receiving cabazitaxel-based chemotherapy.Can Urol Assoc J. 2013 Jan-Feb;7(1-2 Suppl 1):S18-24. doi: 10.5489/cuaj.275. Can Urol Assoc J. 2013. PMID: 23682302 Free PMC article.
-
Metastatic castration-resistant prostate cancer: The emerging continuum of care.Can Urol Assoc J. 2013 Jan-Feb;7(1-2 Suppl 1):S3. doi: 10.5489/cuaj.229. Can Urol Assoc J. 2013. PMID: 23682303 Free PMC article. No abstract available.
References
-
- Canadian Cancer Society Prostate cancer statistics at a glance. Available at: http://www.cancer.ca/canada-wide/about%20cancer/cancer%20statistics/stat.... Accessed October 1, 2012.
-
- National Comprehensive Cancer Network . Prostate cancer. Pennsylvania: NCCN; 2012. NCCN clinical practice guidelines in oncology. Version 3.2012.
-
- Tannock IF, Osoba D, Stockier MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol. 1996;14:1756–64. - PubMed
LinkOut - more resources
Full Text Sources
