Engineering and Applications of DNA-Grafting Polymer Materials
- PMID: 23682309
- PMCID: PMC3652475
- DOI: 10.1039/C2SC21198J
Engineering and Applications of DNA-Grafting Polymer Materials
Abstract
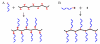
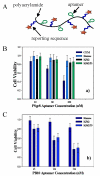
The emergence of hybrid materials combining biomacromolecules and organic polymers has received broad attention based on their potential applications in chemical, biological and materials sciences. Among different coupling strategies, the grafting of oligonucleotides to organic polymers as side chains by covalent bonds provides a novel platform whereby the properties of both oligonucleotides and polymer backbone are integrated, manipulated and optimized for various applications. In this review, we give the perspective on this specific type of DNA polymer hybrid materials , using selected examples with emphasis on bioanalysis, biomedicine and stimuli-responsive materials. It is expected the success of DNA-grafting polymers will not only impact the frabication of novel bimolecule incorporated materials, but also will influence how the properties of synthetic materials are tailored using different functional groups.
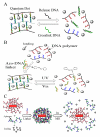
Figures
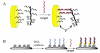
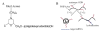

Similar articles
-
Nucleic acid/organic polymer hybrid materials: synthesis, superstructures, and applications.Angew Chem Int Ed Engl. 2010 Nov 8;49(46):8574-87. doi: 10.1002/anie.200906820. Angew Chem Int Ed Engl. 2010. PMID: 20845335 Review.
-
DNA block copolymers: functional materials for nanoscience and biomedicine.Acc Chem Res. 2012 Sep 18;45(9):1419-30. doi: 10.1021/ar200211a. Epub 2012 Jun 22. Acc Chem Res. 2012. PMID: 22726237 Review.
-
Covalent connections between metal-organic frameworks and polymers including covalent organic frameworks.Chem Soc Rev. 2023 Sep 18;52(18):6379-6416. doi: 10.1039/d3cs00302g. Chem Soc Rev. 2023. PMID: 37667818 Review.
-
Engineering responsive polymer building blocks with host-guest molecular recognition for functional applications.Acc Chem Res. 2014 Jul 15;47(7):2084-95. doi: 10.1021/ar5001007. Epub 2014 Apr 17. Acc Chem Res. 2014. PMID: 24742049
-
Planning Implications Related to Sterilization-Sensitive Science Investigations Associated with Mars Sample Return (MSR).Astrobiology. 2022 Jun;22(S1):S112-S164. doi: 10.1089/AST.2021.0113. Epub 2022 May 19. Astrobiology. 2022. PMID: 34904892
Cited by
-
Functional nucleic acid-based hydrogels for bioanalytical and biomedical applications.Chem Soc Rev. 2016 Mar 7;45(5):1410-31. doi: 10.1039/c5cs00586h. Chem Soc Rev. 2016. PMID: 26758955 Free PMC article. Review.
-
Endogenous signalling control of cell adhesion by using aptamer functionalized biocompatible hydrogel.Chem Sci. 2015 Dec 1;6(12):6762-6768. doi: 10.1039/c5sc02565f. Epub 2015 Aug 27. Chem Sci. 2015. PMID: 28757967 Free PMC article.
-
Elucidating Sequence-Assembly Relationships for Bilingual PNA Biopolymers.ACS Omega. 2023 Sep 29;8(40):37442-37450. doi: 10.1021/acsomega.3c05528. eCollection 2023 Oct 10. ACS Omega. 2023. PMID: 37841192 Free PMC article.
-
Automated Synthesis of Well-Defined Polymers and Biohybrids by Atom Transfer Radical Polymerization Using a DNA Synthesizer.Angew Chem Int Ed Engl. 2017 Mar 1;56(10):2740-2743. doi: 10.1002/anie.201611567. Epub 2017 Feb 6. Angew Chem Int Ed Engl. 2017. PMID: 28164438 Free PMC article.
-
A Versatile and Efficient Method to Isolate DNA-Polymer Conjugates.ACS Macro Lett. 2023 Sep 19;12(9):1257-1263. doi: 10.1021/acsmacrolett.3c00371. Epub 2023 Sep 1. ACS Macro Lett. 2023. PMID: 37656875 Free PMC article.
References
-
- Caruthers MH. Acc. Chem. Res. 1991;24:278–284.
-
- Dawson PE, Kent SBH. Annu. Rev. Biochem. 2000;69:923–960. - PubMed
-
- Seeberger PH, Werz DB. Nat. Rev. Drug Discovery. 2005;4:751–763. - PubMed
-
- Seeberger PH, Werz DB. Nature. 2007;446:1046–1051. - PubMed
-
- Deming TJ. Adv. Drug Deliv. Rev. 2002;54:1145–1155. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

