Synthesis of a library of "lead-like" γ-lactams by a one pot, four-component reaction
- PMID: 23682712
- PMCID: PMC3816122
- DOI: 10.1021/co400049f
Synthesis of a library of "lead-like" γ-lactams by a one pot, four-component reaction
Abstract
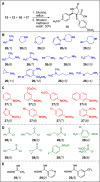
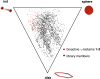
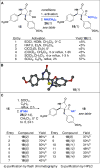
The synthesis of a pilot scale library of 116 structurally diverse γ-lactams is reported. The library core structure emanates from a γ-lactam forming one-pot, four-component reaction of ammonium acetate, p-methoxythiophenol, p-methoxybenzaldehyde, and maleic anhydride. Structural diversity then arises from amide coupling, thioaryl cleavage, N-functionalization, and heterocycle forming reactions on this core structure. Computational analysis reveals that the library contains molecular properties and shape diversity suitable for drug lead and biological probe discovery.
Figures








Similar articles
-
Microwave-assisted diastereoselective two-step three-component synthesis for rapid access to drug-like libraries of substituted 3-amino-β-lactams.Bioorg Med Chem. 2018 Jan 1;26(1):41-49. doi: 10.1016/j.bmc.2017.11.014. Epub 2017 Nov 16. Bioorg Med Chem. 2018. PMID: 29174508
-
Synthesis of 1,4,5 trisubstituted γ-lactams via a 3-component cascade reaction.Bioorg Med Chem. 2015 Jun 1;23(11):2695-8. doi: 10.1016/j.bmc.2015.01.041. Epub 2015 Jan 31. Bioorg Med Chem. 2015. PMID: 25684425
-
Synthesis of a γ-lactam library via formal cycloaddition of imines and substituted succinic anhydrides.ACS Comb Sci. 2012 Mar 12;14(3):218-23. doi: 10.1021/co2001873. Epub 2012 Feb 10. ACS Comb Sci. 2012. PMID: 22225535 Free PMC article.
-
Trifluoromethylated lactams: promising small molecules in the search for effective drugs.Chem Commun (Camb). 2025 Jan 9;61(5):785-802. doi: 10.1039/d4cc05324a. Chem Commun (Camb). 2025. PMID: 39659174 Review.
-
Employing Photocatalysis for the Design and Preparation of DNA-Encoded Libraries: A Case Study.Chem Rec. 2021 Apr;21(4):616-630. doi: 10.1002/tcr.202000148. Epub 2021 Feb 11. Chem Rec. 2021. PMID: 33570227 Review.
Cited by
-
Piperidine Derivatives: Recent Advances in Synthesis and Pharmacological Applications.Int J Mol Sci. 2023 Feb 2;24(3):2937. doi: 10.3390/ijms24032937. Int J Mol Sci. 2023. PMID: 36769260 Free PMC article. Review.
-
Diastereoselective Base-Catalyzed Formal [4 + 2] Cycloadditions of N-Sulfonyl Imines and Cyclic Anhydrides.Org Lett. 2017 May 19;19(10):2466-2469. doi: 10.1021/acs.orglett.7b00468. Epub 2017 May 5. Org Lett. 2017. PMID: 28474515 Free PMC article.
References
-
- Manam RR, Teisan S, White DJ, Nicholson B, Grodberg J, Neuteboom STC, Lam KS, Mosca DA, Lloyd GK, Potts BCM. Lajollamycin, a nitro-tetraene spiro-β-lactone-γ-lactam antibiotic from the marine actinomycete Streptomyces nodosus. J Nat Prod. 2005;68:240–243. - PubMed
-
- Mori T, Takahashi K, Kashiwabara M, Uemura D, Katayama C, Iwadare S, Shizuri Y, Mitomo R, Nakano F, Matsuzaki A. Structure of oxazolomycin, a novel β-lactone antibiotic. Tetrahedron Lett. 1985;26:1073–6.
-
- Feling RH, Buchanan GO, Mincer TJ, Kauffman CA, Jensen PR, Fenical W. Salinosporamide A: a highly cytotoxic proteasome inhibitor from a novel microbial source, a marine bacterium of the new genus Salinospora. Angew Chem Int Ed. 2003;42:355–357. - PubMed
-
- Fenteany G, Standaert RF, Lane WS, Choi S, Corey EJ, Schreiber SL. Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science (Washington, D C) 1995;268:726–31. - PubMed
-
- Guntern A, Ioset JR, Queiroz EF, Sandor P, Foggin CM, Hostettmann K. Heliotropamide, a Novel Oxopyrrolidine-3-carboxamide from Heliotropium ovalifolium. J Nat Prod. 2003;66:1550–1553. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

