Hydrogen sulfide attenuates opioid dependence by suppression of adenylate cyclase/cAMP pathway
- PMID: 23682813
- PMCID: PMC3880902
- DOI: 10.1089/ars.2012.5119
Hydrogen sulfide attenuates opioid dependence by suppression of adenylate cyclase/cAMP pathway
Abstract
Aims: The best-established mechanism of opioid dependence is the up-regulation of adenylate cyclase (AC)/cAMP pathway, which was reported to be negatively regulated by hydrogen sulfide (H2S), a novel endogenous neuromodulator. The present study was, therefore, designed to determine whether H2S is able to attenuate the development of opioid dependence via down-regulating AC/cAMP pathway.
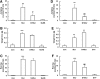
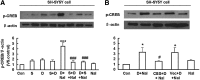
Results: We demonstrated that application of sodium hydrosulphide (NaHS) and GYY4137, two donors of H2S, significantly alleviated naloxone-induced robust withdrawal jumping (the most sensitive and reliable index of opioid physical dependence) in morphine-treated mice. Repeated treatment with NaHS inhibited the up-regulated protein expression of AC in the striatum of morphine-dependent mice. Furthermore, NaHS also attenuated morphine/naloxone-elevated mRNA levels of AC isoform 1 and 8, production of cAMP, and phosphorylation of cAMP response element-binding protein (CREB) in mice striatum. These effects were mimicked by the application of exogenous H2S or over-expression of cystathione-β-synthase, an H2S -producing enzyme, in SH-SY5Y neuronal cells on treatment with [D-Ala(2),N-Me-Phe(4),Gly(5)-ol]-Enkephalin, a selective μ-opioid receptor agonist. Blockade of extracellular-regulated protein kinase 1/2 (ERK1/2) with its specific inhibitor attenuated naloxone-induced CREB phosphorylation. Pretreatment with NaHS or stimulation of endogenous H2S production also significantly suppressed opioid withdrawal-induced ERK1/2 activation in mice striatum or SH-SY5Y cells.
Innovation: H2S treatment is important in prevention of the development of opioid dependence via suppression of cAMP pathway in both animal and cellular models.
Conclusion: Our data suggest a potential role of H2S in attenuating the development of opioid dependence, and the underlying mechanism is closely related to the inhibition of AC/cAMP pathway.
Figures







Similar articles
-
Hydrogen sulfide inhibits opioid withdrawal-induced pain sensitization in rats by down-regulation of spinal calcitonin gene-related peptide expression in the spine.Int J Neuropsychopharmacol. 2014 Sep;17(9):1387-95. doi: 10.1017/S1461145714000583. Epub 2014 May 13. Int J Neuropsychopharmacol. 2014. PMID: 24824948
-
Exogenous sodium hydrosulfide can attenuate naloxone-precipitated withdrawal syndromes and affect cAMP signaling pathway in heroin-dependent rat's nucleus accumbens.Eur Rev Med Pharmacol Sci. 2012 Dec;16(14):1974-82. Eur Rev Med Pharmacol Sci. 2012. PMID: 23242725
-
Opioid-induced mitogen-activated protein kinase signaling in rat enteric neurons following chronic morphine treatment.PLoS One. 2014 Oct 10;9(10):e110230. doi: 10.1371/journal.pone.0110230. eCollection 2014. PLoS One. 2014. PMID: 25302800 Free PMC article.
-
Reflections on: "A general role for adaptations in G-Proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function".Brain Res. 2016 Aug 15;1645:71-4. doi: 10.1016/j.brainres.2015.12.039. Epub 2015 Dec 29. Brain Res. 2016. PMID: 26740398 Free PMC article. Review.
-
Neuroprotective Methodologies in the Treatment of Multiple Sclerosis Current Status of Clinical and Pre-clinical Findings.Curr Drug Discov Technol. 2021;18(1):31-46. doi: 10.2174/1570163817666200207100903. Curr Drug Discov Technol. 2021. PMID: 32031075 Review.
Cited by
-
Synthesis of the Mechanisms of Opioid Tolerance: Do We Still Say NO?Cell Mol Neurobiol. 2021 Jul;41(5):927-948. doi: 10.1007/s10571-021-01065-8. Epub 2021 Mar 11. Cell Mol Neurobiol. 2021. PMID: 33704603 Free PMC article. Review.
-
The cystathionine-γ-lyase inhibitor DL-propargylglycine augments the ability of L-cysteine ethyl ester to overcome the adverse effects of morphine on breathing.Am J Physiol Lung Cell Mol Physiol. 2025 Jun 1;328(6):L809-L825. doi: 10.1152/ajplung.00003.2025. Epub 2025 Mar 18. Am J Physiol Lung Cell Mol Physiol. 2025. PMID: 40099842 Free PMC article.
-
Sodium/calcium exchanger is upregulated by sulfide signaling, forms complex with the β1 and β3 but not β2 adrenergic receptors, and induces apoptosis.Pflugers Arch. 2014 Jul;466(7):1329-42. doi: 10.1007/s00424-013-1366-1. Epub 2013 Oct 10. Pflugers Arch. 2014. PMID: 24114174
-
Age-Dependent, Subunit Specific Action of Hydrogen Sulfide on GluN1/2A and GluN1/2B NMDA Receptors.Front Cell Neurosci. 2017 Nov 24;11:375. doi: 10.3389/fncel.2017.00375. eCollection 2017. Front Cell Neurosci. 2017. PMID: 29225568 Free PMC article.
-
Synaptic Structure and Transcriptomic Profiling of Reward and Sensory Brain Areas in Male Mice of Fentanyl Addiction.Subst Abuse Rehabil. 2024 Dec 6;15:233-245. doi: 10.2147/SAR.S484167. eCollection 2024. Subst Abuse Rehabil. 2024. PMID: 39659661 Free PMC article.
References
-
- Chakrabarti S, Rivera M, Yan SZ, Tang WJ, and Gintzler AR. Chronic morphine augments G(beta)(gamma)/Gs(alpha) stimulation of adenylyl cyclase: relevance to opioid tolerance. Mol Pharmacol 54: 655–662, 1998 - PubMed
-
- Chakrabarti S, Wang L, Tang WJ, and Gintzler AR. Chronic morphine augments adenylyl cyclase phosphorylation: relevance to altered signaling during tolerance/dependence. Mol Pharmacol 54: 949–953, 1998 - PubMed
-
- Chan JS, Chiu TT, and Wong YH. Activation of type II adenylyl cyclase by the cloned mu-opioid receptor: coupling to multiple G proteins. J Neurochem 65: 2682–2689, 1995 - PubMed
-
- Chao JR, Ni YG, Bolanos CA, Rahman Z, DiLeone RJ, and Nestler EJ. Characterization of the mouse adenylyl cyclase type VIII gene promoter: regulation by cAMP and CREB. Eur J Neurosci 16: 1284–1294, 2002 - PubMed
-
- Crain SM. and Shen KF. Ultra-low concentrations of naloxone selectively antagonize excitatory effects of morphine on sensory neurons, thereby increasing its antinociceptive potency and attenuating tolerance/dependence during chronic cotreatment. Proc Natl Acad Sci U S A 92: 10540–10544, 1995 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

