Nox family NADPH oxidases in mechano-transduction: mechanisms and consequences
- PMID: 23682993
- PMCID: PMC3924808
- DOI: 10.1089/ars.2013.5414
Nox family NADPH oxidases in mechano-transduction: mechanisms and consequences
Abstract
Significance: The majority of cells in a multi-cellular organism are continuously exposed to ever-changing physical forces. Mechano-transduction links these events to appropriate reactions of the cells involving stimulation of signaling cascades, reorganization of the cytoskeleton and alteration of gene expression.
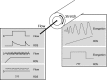
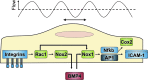
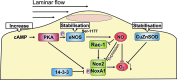
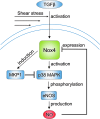
Recent advances: Mechano-transduction alters the cellular redox balance and the formation of reactive oxygen species (ROS). Nicotine amide adenine dinucleotide reduced form (NADPH) oxidases of the Nox family are prominent ROS generators and thus, contribute to this stress-induced ROS formation.
Critical issues: Different types and patterns of mechano-stress lead to Nox-dependent ROS formation and Nox-mediated ROS formation contributes to cellular responses and adaptation to physical forces. Thereby, Nox enzymes can mediate vascular protection during physiological mechano-stress. Despite this, over-activation and induction of Nox enzymes and a subsequent substantial increase in ROS formation also promotes oxidative stress in pathological situations like disturbed blood flow or extensive stretch.
Future directions: Individual protein targets of Nox-mediated redox-signaling will be identified to better understand the specificity of Nox-dependent ROS signaling in mechano-transduction. Nox-inhibitors will be tested to reduce cellular activation in response to mechano-stimuli.
Figures


References
-
- Abe M, O'Connor P, Kaldunski M, Liang M, Roman RJ, and Cowley AW, Jr., Effect of sodium delivery on superoxide and nitric oxide in the medullary thick ascending limb. Am J Physiol Renal Physiol 291: F350–F357, 2006 - PubMed
-
- Adams V, Linke A, Krankel N, Erbs S, Gielen S, Mobius-Winkler S, Gummert JF, Mohr FW, Schuler G, and Hambrecht R. Impact of regular physical activity on the NAD(P)H oxidase and angiotensin receptor system in patients with coronary artery disease. Circulation 111: 555–562, 2005 - PubMed
-
- Agarwal U, Zhou X, Weber K, Dadabayev AR, and Penn MS. Critical role for white blood cell NAD(P)H oxidase-mediated plasminogen activator inhibitor-1 oxidation and ventricular rupture following acute myocardial infarction. J Mol Cell Cardiol 50: 426–432, 2011 - PubMed
-
- Ambasta RK, Schreiber JG, Janiszewski M, Busse R, and Brandes RP. Noxa1 is a central component of the smooth muscle NADPH oxidase in mice. Free Radic Biol Med 41: 193–201, 2006 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
