Tryptophan cluster protects human γD-crystallin from ultraviolet radiation-induced photoaggregation in vitro
- PMID: 23683003
- PMCID: PMC3823069
- DOI: 10.1111/php.12096
Tryptophan cluster protects human γD-crystallin from ultraviolet radiation-induced photoaggregation in vitro
Abstract
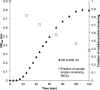
Exposure to ultraviolet radiation (UVR) is a significant risk factor for age-related cataract, a disease of the human lens and the most prevalent cause of blindness in the world. Cataract pathology involves protein misfolding and aggregation of the primary proteins of the lens, the crystallins. Human γD-crystallin (HγD-Crys) is a major γ-crystallin in the nucleus of the human lens. We report here analysis of UVR-induced damage to HγD-Crys in vitro. Irradiation of solutions of recombinant HγD-Crys with UVA/UVB light produced a rise in solution turbidity due to polymerization of the monomeric crystallins into higher molecular weight aggregates. A significant fraction of this polymerized protein was covalently linked. Photoaggregation of HγD-Crys required oxygen and its rate was protein concentration and UVR dose dependent. To investigate the potential roles of individual tryptophan residues in photoaggregation, triple W:F mutants of HγD-Crys were irradiated. Surprisingly, despite reducing UVR absorbing capacity, multiple W:F HγD-Crys mutant proteins photoaggregated more quickly and extensively than wild type. The results reported here are consistent with previous studies that postulated that an energy transfer mechanism between the highly conserved pairs of tryptophan residues in HγD-Crys could be protective against UVR-induced photodamage.
© 2013 The Authors. Photochemistry and Photobiology published by Wiley Periodicals, Inc. on behalf of The American Society of Photobiology.
Figures







Similar articles
-
Tyrosine/cysteine cluster sensitizing human γD-crystallin to ultraviolet radiation-induced photoaggregation in vitro.Biochemistry. 2014 Feb 18;53(6):979-90. doi: 10.1021/bi401397g. Epub 2014 Feb 5. Biochemistry. 2014. PMID: 24410332 Free PMC article.
-
Probing folding and fluorescence quenching in human gammaD crystallin Greek key domains using triple tryptophan mutant proteins.Protein Sci. 2004 Aug;13(8):2223-35. doi: 10.1110/ps.04627004. Protein Sci. 2004. PMID: 15273315 Free PMC article.
-
In vitro unfolding, refolding, and polymerization of human gammaD crystallin, a protein involved in cataract formation.Protein Sci. 2003 Mar;12(3):480-90. doi: 10.1110/ps.0225503. Protein Sci. 2003. PMID: 12592018 Free PMC article.
-
Mechanism of the highly efficient quenching of tryptophan fluorescence in human gammaD-crystallin.Biochemistry. 2006 Sep 26;45(38):11552-63. doi: 10.1021/bi060988v. Biochemistry. 2006. PMID: 16981715
-
Inhibition of unfolding and aggregation of lens protein human gamma D crystallin by sodium citrate.Exp Eye Res. 2011 Oct;93(4):371-81. doi: 10.1016/j.exer.2011.04.011. Epub 2011 May 12. Exp Eye Res. 2011. PMID: 21600897 Free PMC article.
Cited by
-
Characterization of βB2-crystallin tryptophan mutants reveals two different folding states in solution.Protein Sci. 2024 Jul;33(7):e5092. doi: 10.1002/pro.5092. Protein Sci. 2024. PMID: 38924206 Free PMC article.
-
Late Embryogenesis Abundant Proteins Contribute to the Resistance of Toxoplasma gondii Oocysts against Environmental Stresses.mBio. 2023 Apr 25;14(2):e0286822. doi: 10.1128/mbio.02868-22. Epub 2023 Feb 21. mBio. 2023. PMID: 36809045 Free PMC article.
-
Significance of Singlet Oxygen Molecule in Pathologies.Int J Mol Sci. 2023 Feb 1;24(3):2739. doi: 10.3390/ijms24032739. Int J Mol Sci. 2023. PMID: 36769060 Free PMC article. Review.
-
An ultraviolet-driven rescue pathway for oxidative stress to eye lens protein human gamma-D crystallin.Commun Chem. 2024 Apr 10;7(1):81. doi: 10.1038/s42004-024-01163-w. Commun Chem. 2024. PMID: 38600176 Free PMC article.
-
The βγ-crystallins: native state stability and pathways to aggregation.Prog Biophys Mol Biol. 2014 Jul;115(1):32-41. doi: 10.1016/j.pbiomolbio.2014.05.002. Epub 2014 May 14. Prog Biophys Mol Biol. 2014. PMID: 24835736 Free PMC article. Review.
References
-
- Stefani M, Dobson CM. Protein aggregation and aggregate toxicity: New insights into protein folding, misfolding diseases and biological evolution. J. Mol. Med. 2003;81:678–699. - PubMed
-
- Congdon N, Vingerling JR, Klein BEK, West S, Friedman DS, Kempen J, O'Colmain B, Wu S-Y, Taylor HR, Eye Diseases Prevalence Research Group Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch. Ophthalmol. (Chicago, IL, U.S.) 2004;122:487–494. - PubMed
-
- Abraham A, Condon N, Gower EW. The new epidemiology of cataract. Ophthalmol. Clin. North America. 2006;19:415–425. - PubMed
-
- Cockell CS. Biological effects of high ultraviolet radiation on early earth—A theoretical evaluation. J. Theor. Biol. 1998;193:717–729. - PubMed
-
- Lagerwerf S, Vrouwe MG, Overmeer RM, Fousteri MI, Mullenders LHF. DNA damage response and transcription. DNA Repair. 2011;10:743–750. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

