Activation of p53 with Nutlin-3a radiosensitizes lung cancer cells via enhancing radiation-induced premature senescence
- PMID: 23683497
- PMCID: PMC3739976
- DOI: 10.1016/j.lungcan.2013.04.017
Activation of p53 with Nutlin-3a radiosensitizes lung cancer cells via enhancing radiation-induced premature senescence
Abstract
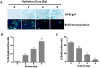
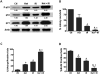
Radiotherapy is routinely used for the treatment of lung cancer. However, the mechanisms underlying ionizing radiation (IR)-induced senescence and its role in lung cancer treatment are poorly understood. Here, we show that IR suppresses the proliferation of human non-small cell lung cancer (NSCLC) cells via an apoptosis-independent mechanism. Further investigations reveal that the anticancer effect of irradiation correlates well with IR-induced premature senescence, as evidenced by increased senescence-associated β-glactosidase (SA-β-gal) staining, decreased BrdU incorporation and elevated expression of p16(INK4a) (p16) in irradiated NSCLC cells. Mechanistic studies indicate that the induction of senescence is associated with activation of the p53-p21 pathway, and that inhibition of p53 transcriptional activity by PFT-α attenuates IR-induced tumor cell killing and senescence. Gain-of-function assays demonstrate that restoration of p53 expression sensitizes H1299 cells to irradiation, whereas knockdown of p53 expression by siRNA inhibits IR-induced senescence in H460 cells. Furthermore, treatment with Nutlin-3a, a small molecule inhibitor of MDM2, enhances IR-induced tumor cell killing and senescence by stabilizing the activation of the p53-p21 signaling pathway. Taken together, these findings demonstrate for the first time that pharmacological activation of p53 by Nutlin-3a can sensitize lung cancer cells to radiation therapy via promoting IR-induced premature senescence.
Keywords: Non-small cell lung cancer; Nutilin-3a; Radiotherapy; Senescence; p53; siRNA.
Copyright © 2013 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
Figures



Similar articles
-
Activation of p53 by Nutlin-3a, an antagonist of MDM2, induces apoptosis and cellular senescence in adult T-cell leukemia cells.Leukemia. 2009 Nov;23(11):2090-101. doi: 10.1038/leu.2009.171. Epub 2009 Aug 27. Leukemia. 2009. PMID: 19710698
-
Activation of p53 by nutlin-3a induces apoptosis and cellular senescence in human glioblastoma multiforme.PLoS One. 2011 Apr 5;6(4):e18588. doi: 10.1371/journal.pone.0018588. PLoS One. 2011. PMID: 21483692 Free PMC article.
-
Resveratrol enhances ionizing radiation-induced premature senescence in lung cancer cells.Int J Oncol. 2013 Dec;43(6):1999-2006. doi: 10.3892/ijo.2013.2141. Epub 2013 Oct 17. Int J Oncol. 2013. PMID: 24141489 Free PMC article.
-
Nutlins and ionizing radiation in cancer therapy.Curr Pharm Des. 2010;16(12):1427-42. doi: 10.2174/138161210791033932. Curr Pharm Des. 2010. PMID: 20166982 Review.
-
Radiosensitization of prostate cancer by priming the wild-type p53-dependent cellular senescence pathway.Cancer Biol Ther. 2007 Aug;6(8):1165-70. doi: 10.4161/cbt.6.8.4544. Epub 2007 Aug 5. Cancer Biol Ther. 2007. PMID: 18059157 Free PMC article. Review.
Cited by
-
P53 together with ferroptosis: a promising strategy leaving cancer cells without escape.Acta Biochim Biophys Sin (Shanghai). 2024 Jan 25;56(1):1-14. doi: 10.3724/abbs.2023270. Acta Biochim Biophys Sin (Shanghai). 2024. PMID: 38105650 Free PMC article.
-
Cancer treatment-induced NAD+ depletion in premature senescence and late cardiovascular complications.J Cardiovasc Aging. 2022;2:28. doi: 10.20517/jca.2022.13. Epub 2022 Apr 29. J Cardiovasc Aging. 2022. PMID: 35801078 Free PMC article.
-
Oxidative stress induces senescence in breast cancer stem cells.Biochem Biophys Res Commun. 2019 Jul 5;514(4):1204-1209. doi: 10.1016/j.bbrc.2019.05.098. Epub 2019 May 17. Biochem Biophys Res Commun. 2019. PMID: 31109646 Free PMC article.
-
Modulating Both Tumor Cell Death and Innate Immunity Is Essential for Improving Radiation Therapy Effectiveness.Front Immunol. 2017 May 26;8:613. doi: 10.3389/fimmu.2017.00613. eCollection 2017. Front Immunol. 2017. PMID: 28603525 Free PMC article. Review.
-
Crosstalk between the IGF-1R/AKT/mTORC1 pathway and the tumor suppressors p53 and p27 determines cisplatin sensitivity and limits the effectiveness of an IGF-1R pathway inhibitor.Oncotarget. 2016 May 10;7(19):27511-26. doi: 10.18632/oncotarget.8484. Oncotarget. 2016. PMID: 27050276 Free PMC article.
References
-
- Elmore LW, Rehder CW, Di X, McChesney PA, Jackson-Cook CK, et al. Adriamycin-induced senescence in breast tumor cells involves functional p53 and telomere dysfunction. J Biol Chem. 2002;277:35509–15. - PubMed
-
- te Poele RH, Okorokov AL, Jardine L, Cummings J, Joel SP. DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res. 2002;62:1876–83. - PubMed
-
- Rebbaa A, Zheng X, Chou PM, Mirkin BL. Caspase inhibition switches doxorubicin-induced apoptosis to senescence. Oncogene. 2003;22:2805–11. - PubMed
-
- Yin DX, Schimke RT. BCL-2 expression delays drug-induced apoptosis but does not increase clonogenic survival after drug treatment in HeLa cells. Cancer Res. 1995;55:4922–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

