Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial
- PMID: 23683600
- PMCID: PMC4083737
- DOI: 10.1016/S0140-6736(13)60856-9
Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial
Abstract
Background: Osteoporosis medications increase bone-mineral density (BMD) and lower but do not eliminate fracture risk. The combining of anabolic agents with bisphosphonates has not improved efficacy. We compared combined teriparatide and denosumab with both agents alone.
Methods: From September, 2009, to January, 2011, we enrolled postmenopausal women with osteoporosis into this randomised, controlled trial. Patients were assigned in a 1:1:1 ratio to receive 20 μg teriparatide daily, 60 mg denosumab every 6 months, or both. BMD was measured at 0, 3, 6, and 12 months. Women who completed at least one study visit after baseline were assessed in a modified intention-to-treat analysis. This trial is registered with ClinicalTrials.gov, number NCT00926380.
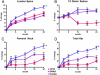
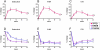
Findings: 94 (94%) of 100 eligible women completed at least one study visit after baseline. At 12 months, posterior-anterior lumbar spine BMD increased more in the combination group (9·1%, [SD 3·9]) than in the teriparatide (6·2% [4·6], p=0·0139) or denosumab (5·5% [3·3], p=0·0005) groups. Femoral-neck BMD also increased more in the combination group (4·2% [3·0]) than in the teriparatide (0·8% [4·1], p=0·0007) and denosumab (2·1% [3·8], p=0·0238) groups, as did total-hip BMD (combination, 4·9% [2·9]; teriparatide, 0·7% [2·7], p<0·0001; denosumab 2·5% [2·6], p=0·0011).
Interpretation: Combined teriparatide and denosumab increased BMD more than either agent alone and more than has been reported with approved therapies. Combination treatment might, therefore, be useful to treat patients at high risk of fracture.
Funding: Amgen, Eli Lilly, National Center for Research Resources.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

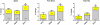
Comment in
-
Is it time to combine osteoporosis therapies?Lancet. 2013 Jul 6;382(9886):5-7. doi: 10.1016/S0140-6736(13)60984-8. Epub 2013 May 15. Lancet. 2013. PMID: 23683601 Free PMC article.
-
Osteoporosis: Teriparatide and denosumab: two drugs are better than one!Nat Rev Rheumatol. 2013 Jul;9(7):383. doi: 10.1038/nrrheum.2013.79. Epub 2013 May 28. Nat Rev Rheumatol. 2013. PMID: 23712125 No abstract available.
-
Therapy: raising BMD-two drugs are better than one.Nat Rev Endocrinol. 2013 Aug;9(8):442. doi: 10.1038/nrendo.2013.112. Epub 2013 May 28. Nat Rev Endocrinol. 2013. PMID: 23712247 No abstract available.
-
[Combination therapy in postmenopausal osteoporosis leads to denser bones than monotherapy].Praxis (Bern 1994). 2013 Sep 18;102(19):1203-4. doi: 10.1024/1661-8157/a001403. Praxis (Bern 1994). 2013. PMID: 24025179 German. No abstract available.
-
Oséoporose: tériparatide + dénosumab augmentent plus la densité minérale osseuse que chacun des médicaments adminitsré seul.Rev Prat. 2013 Sep;63(7):924. Rev Prat. 2013. PMID: 24167890 French. No abstract available.
References
-
- Reszka AA, Rodan GA. Mechanism of action of bisphosphonates. Curr Osteoporos Rep. 2003;1:45–52. - PubMed
-
- Delmas PD. Clinical potential of RANKL inhibition for the management of postmenopausal osteoporosis and other metabolic bone diseases. J Clin Densitom. 2008;11:325–338. - PubMed
-
- Dempster DW, Zhou H, Recker RR, et al. Skeletal histomorphometry in subjects on teriparatide or zoledronic acid therapy (SHOTZ) study: a randomized controlled trial. J Clin Endocrinol Metab. 2012;97:2799–2808. - PubMed
-
- Compston J. The use of combination therapy in the treatment of postmenopausal osteoporosis. Endocrine. 2012;41:11–18. - PubMed
-
- Black DM, Greenspan SL, Ensrud KE, et al. The effects of parathyroid hormone and alendronate alone or in combination in post-menopausal osteoporosis. N Engl J Med. 2003;349:1207–1215. - PubMed

