Role of prefrontal cortex glucocorticoid receptors in stress and emotion
- PMID: 23683655
- PMCID: PMC3797253
- DOI: 10.1016/j.biopsych.2013.03.024
Role of prefrontal cortex glucocorticoid receptors in stress and emotion
Abstract
Background: Stress-related disorders (e.g., depression) are associated with hypothalamic-pituitary-adrenocortical axis dysregulation and prefrontal cortex (PFC) dysfunction, suggesting a functional link between aberrant prefrontal corticosteroid signaling and mood regulation.
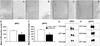
Methods: We used a virally mediated knockdown strategy (short hairpin RNA targeting the glucocorticoid receptor [GR]) to attenuate PFC GR signaling in the rat PFC. Adult male rats received bilateral microinjections of vector control or short hairpin RNA targeting the GR into the prelimbic (n = 44) or infralimbic (n = 52) cortices. Half of the animals from each injection group underwent chronic variable stress, and all were subjected to novel restraint. The first 2 days of chronic variable stress were used to assess depression- and anxiety-like behavior in the forced swim test and open field.
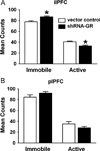
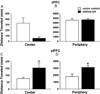
Results: The GR knockdown confined to the infralimbic PFC caused acute stress hyper-responsiveness, sensitization of stress responses after chronic variable stress, and induced depression-like behavior (increased immobility in the forced swim test). Knockdown of GR in the neighboring prelimbic PFC increased hypothalamic-pituitary-adrenocortical axis responses to acute stress and caused hyperlocomotion in the open field, but did not affect stress sensitization or helplessness behavior.
Conclusions: The data indicate a marked functional heterogeneity of glucocorticoid action in the PFC and highlight a prominent role for the infralimbic GR in appropriate stress adaptation, emotional control, and mood regulation.
Keywords: Depression-like behavior; HPA axis; glucocorticoid receptor; prefrontal cortex; rat; stress.
© 2013 Society of Biological Psychiatry.
Conflict of interest statement
The authors report no biomedical financial interests or potential conflicts of interest.
Figures



References
-
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. - PubMed
-
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. - PubMed
-
- Reul JM, De Kloet ER. Anatomical resolution of two types of corticosterone receptor sites in rat brain with in vitro autoradiography and computerized image analysis. J Steroid Biochem. 1986;24:269–272. - PubMed
-
- Fuxe K, Wikstrom AC, Okret S, Agnati LF, Harfstrand A, Yu ZY, et al. Mapping of glucocorticoid receptor immunoreactive neurons in the rat tel- and diencephalon using a monoclonal antibody against rat liver glucocorticoid receptor. Endocrinology. 1985;117:1803–1812. - PubMed
-
- Meaney MJ, Sapolsky RM, Aitken DH, McEwen BS. [3H] dexamethasone binding in the limbic brain of the fetal rat. Brain Res. 1985;355:297–300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

