The pathogenesis of Epstein-Barr virus persistent infection
- PMID: 23683686
- PMCID: PMC3789532
- DOI: 10.1016/j.coviro.2013.04.005
The pathogenesis of Epstein-Barr virus persistent infection
Abstract
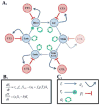
Epstein-Barr virus (EBV) maintains a lifelong infection. According to the germinal center model (GCM), latently infected B cells transit the germinal center (GC) to become resting memory cells. Here, the virus resides quiescently, occasionally reactivating to infect new B cells, completing the cycle of infection. The GCM remains the only model that explains EBV biology and the pathogenesis of lymphoma. Recent work suggests modifications to the model notably that the virus contributes only modestly to the GC process and predictions from mathematical models that quiescence within memory B cells shapes the overall structure of viral infection but is not essential for persistence. Rather, it is the cycle of infection which allows viral persistence at the very low levels observed.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures

References
-
- Babcock GJ, Decker LL, Volk M, Thorley-Lawson DA. EBV persistence in memory B cells in vivo. Immunity. 1998;9(3):395–404. - PubMed
-
- Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med. 2004;350(13):1328–1337. - PubMed
-
- Thorley-Lawson DA. Epstein-Barr virus: exploiting the immune system. Nat Rev Immunol. 2001;1(1):75–82. - PubMed
-
- Babcock GJ, Hochberg D, Thorley-Lawson AD. The expression pattern of Epstein-Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity. 2000;13(4):497–506. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

