Iron in infection and immunity
- PMID: 23684303
- PMCID: PMC3676888
- DOI: 10.1016/j.chom.2013.04.010
Iron in infection and immunity
Abstract
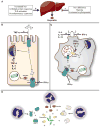
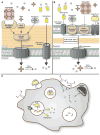
Iron is an essential nutrient for both humans and pathogenic microbes. Because of its ability to exist in one of two oxidation states, iron is an ideal redox catalyst for diverse cellular processes including respiration and DNA replication. However, the redox potential of iron also contributes to its toxicity; thus, iron concentration and distribution must be carefully controlled. Given the absolute requirement for iron by virtually all human pathogens, an important facet of the innate immune system is to limit iron availability to invading microbes in a process termed nutritional immunity. Successful human pathogens must therefore possess mechanisms to circumvent nutritional immunity in order to cause disease. In this review, we discuss regulation of iron metabolism in the setting of infection and delineate strategies used by human pathogens to overcome iron-withholding defenses.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

