Malaria-infected erythrocyte-derived microvesicles mediate cellular communication within the parasite population and with the host immune system
- PMID: 23684304
- PMCID: PMC3687518
- DOI: 10.1016/j.chom.2013.04.009
Malaria-infected erythrocyte-derived microvesicles mediate cellular communication within the parasite population and with the host immune system
Abstract
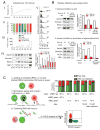
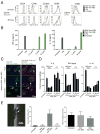
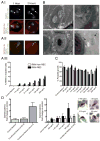
Humans and mice infected with different Plasmodium strains are known to produce microvesicles derived from the infected red blood cells (RBCs), denoted RMVs. Studies in mice have shown that RMVs are elevated during infection and have proinflammatory activity. Here we present a detailed characterization of RMV composition and function in the human malaria parasite Plasmodium falciparum. Proteomics profiling revealed the enrichment of multiple host and parasite proteins, in particular of parasite antigens associated with host cell membranes and proteins involved in parasite invasion into RBCs. RMVs are quantitatively released during the asexual parasite cycle prior to parasite egress. RMVs demonstrate potent immunomodulatory properties on human primary macrophages and neutrophils. Additionally, RMVs are internalized by infected red blood cells and stimulate production of transmission stage parasites in a dose-dependent manner. Thus, RMVs mediate cellular communication within the parasite population and with the host innate immune system.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

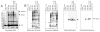


Comment in
-
Talking to each other to initiate sexual differentiation.Cell. 2013 May 23;153(5):945-7. doi: 10.1016/j.cell.2013.05.013. Cell. 2013. PMID: 23706733
-
Parasite biology: Let's talk about sex.Nat Rev Microbiol. 2013 Jul;11(7):433. doi: 10.1038/nrmicro3063. Nat Rev Microbiol. 2013. PMID: 23765082 No abstract available.
-
Malaria: Sensing when it's time for sex.Nature. 2013 Jul 4;499(7456):38-40. doi: 10.1038/499038a. Nature. 2013. PMID: 23823791 No abstract available.
References
-
- Abu Bakar N, Klonis N, Hanssen E, Chan C, Tilley L. Digestive-vacuole genesis and endocytic processes in the early intraerythrocytic stages of Plasmodium falciparum. Journal of cell science. 2010;123:441–450. - PubMed
-
- Alano P. Plasmodium falciparum gametocytes: still many secrets of a hidden life. Molecular microbiology. 2007;66:291–302. - PubMed
-
- Blisnick T, Morales Betoulle ME, Barale JC, Uzureau P, Berry L, Desroses S, Fujioka H, Mattei D, Braun Breton C. Pfsbp1, a Maurer’s cleft Plasmodium falciparum protein, is associated with the erythrocyte skeleton. Molecular and biochemical parasitology. 2000;111:107–121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

