DNA repair mechanisms in dividing and non-dividing cells
- PMID: 23684800
- PMCID: PMC3720834
- DOI: 10.1016/j.dnarep.2013.04.015
DNA repair mechanisms in dividing and non-dividing cells
Abstract
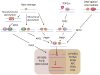
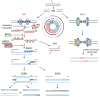
DNA damage created by endogenous or exogenous genotoxic agents can exist in multiple forms, and if allowed to persist, can promote genome instability and directly lead to various human diseases, particularly cancer, neurological abnormalities, immunodeficiency and premature aging. To avoid such deleterious outcomes, cells have evolved an array of DNA repair pathways, which carry out what is typically a multiple-step process to resolve specific DNA lesions and maintain genome integrity. To fully appreciate the biological contributions of the different DNA repair systems, one must keep in mind the cellular context within which they operate. For example, the human body is composed of non-dividing and dividing cell types, including, in the brain, neurons and glial cells. We describe herein the molecular mechanisms of the different DNA repair pathways, and review their roles in non-dividing and dividing cells, with an eye toward how these pathways may regulate the development of neurological disease.
Keywords: 6-4PPs; 8-oxoguanine DNA glycosylase; AOA1; AP; AP endonuclease 1; APE1; APTX; ATM; CPDs; CS; CSR; Cockayne syndrome; DAR; DNA double strand break repair; DNA polymerase β; DNA repair; DNA single strand break repair; DNA single strand breaks; DNA-PKcs; DNA-dependent protein kinase catalytic subunit; DSBR; Dividing and non-dividing; ERCC1; Endogenous DNA damage; FEN1; GG-NER; HNPCC; HR; IR; MAP; MCSZ; MGMT; MMR; MPG; MUTYH; MUTYH-associated polyposis; N-methylpurine-DNA glycosylase; NEIL1; NER; NHEJ; NSC; NTH1; Neural cells; Neurological disorder; O(6)-methylguanine-DNA methyltransferase; OGG1; PARP1; PCNA; PG; PNKP; PUA; Pol β; RFC; RNA polymerase; RNAP; RPA; SCAN1; SCID; SDSA; SSA; SSBR; SSBs; TC-NER; TDP1; TFIIH; TOP1; TTD; Top1 cleavage complex; Top1cc; UNG; X-ray repair cross-complementing protein 1; XP; XRCC1; aprataxin; apurinic/apyrimidinic; ataxia telangiectasia mutated; ataxia with ocular motor apraxia 1; class switch recombination; cyclobutane pyrimidine dimers; dRP; deoxyribose-5-phosphate; endonuclease III-like 1; endonuclease VIII-like 1; excision repair cross complementing 1; flap endonuclease 1; global genome-NER; hereditary nonpolyposis colorectal cancer; homologous recombination; human mutY homolog; ionizing radiation; microcephaly with early-onset, intractable seizures and developmental delay; mismatch repair; neural stem cells; nonhomologous end joining; nucleotide excision repair; phospho-α,β-unsaturated aldehyde; phosphoglycolate; poly(ADP-ribose) polymerase-1; polynucleotide kinase 3′-phosphatase; proliferating cellular nuclear antigen; pyrimidine-(6,4)-pyrimidone photoproducts.; replication factor C; replication protein A; severe combined immunodeficient; single-strand annealing; spinocerebellar ataxia with axonal neuropathy-1; synthesis-dependent strand annealing; topoisomerase 1; transcription domains-associated repair; transcription factor II H; transcription-coupled NER; trichothiodystrophy; tyrosyl-DNA phosphodiesterase 1; uracil-DNA glycosylase; xeroderma pigmentosum.
Published by Elsevier B.V.
Figures


References
-
- Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362:709–715. - PubMed
-
- Hoeijmakers JH. DNA damage, aging, and cancer. N Engl J Med. 2009;361:1475–1485. - PubMed
-
- Rass U, Ahel I, West SC. Defective DNA repair and neurodegenerative disease. Cell. 2007;130:991–1004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

