Sleep active cortical neurons expressing neuronal nitric oxide synthase are active after both acute sleep deprivation and chronic sleep restriction
- PMID: 23685166
- PMCID: PMC3801181
- DOI: 10.1016/j.neuroscience.2013.05.013
Sleep active cortical neurons expressing neuronal nitric oxide synthase are active after both acute sleep deprivation and chronic sleep restriction
Abstract
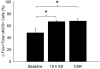
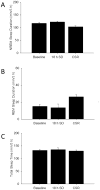
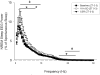
Non-rapid eye movement (NREM) sleep electroencephalographic (EEG) delta power (~0.5-4 Hz), also known as slow wave activity (SWA), is typically enhanced after acute sleep deprivation (SD) but not after chronic sleep restriction (CSR). Recently, sleep-active cortical neurons expressing neuronal nitric oxide synthase (nNOS) were identified and associated with enhanced SWA after short acute bouts of SD (i.e., 6h). However, the relationship between cortical nNOS neuronal activity and SWA during CSR is unknown. We compared the activity of cortical neurons expressing nNOS (via c-Fos and nNOS immuno-reactivity, respectively) and sleep in rats in three conditions: (1) after 18-h of acute SD; (2) after five consecutive days of sleep restriction (SR) (18-h SD per day with 6h ad libitum sleep opportunity per day); (3) and time-of-day matched ad libitum sleep controls. Cortical nNOS neuronal activity was enhanced during sleep after both 18-h SD and 5 days of SR treatments compared to control treatments. SWA and NREM sleep delta energy (the product of NREM sleep duration and SWA) were positively correlated with enhanced cortical nNOS neuronal activity after 18-h SD but not 5days of SR. That neurons expressing nNOS were active after longer amounts of acute SD (18h vs. 6h reported in the literature) and were correlated with SWA further suggest that these cells might regulate SWA. However, since these neurons were active after CSR when SWA was not enhanced, these findings suggest that mechanisms downstream of their activation are altered during CSR.
Keywords: ANOVA; CSR; EEG; EMG; NREM; PBS; REM; SD; SR; SWA; ZT; analysis of variance; c-Fos; chronic sleep restriction; electroencephalogram; electromyogram; nNOS; neuronal nitric oxide synthase; non-rapid eye movement; phosphate-buffered saline; rapid eye movement; sleep deprivation; sleep restriction; slow wave activity; zeitgeber time.
Published by Elsevier Ltd.
Figures


References
-
- Achermann P, Borbély AA. Mathematical models of sleep regulation. Front Biosci. 2003;8:S683–S693. - PubMed
-
- Barf RP, Van Dijk G, Scheurink AJ, Hoffmann K, Novati A, Hulshof HJ, Fuchs E, Meerlo P. Metabolic consequences of chronic sleep restriction in rats: changes in body weight regulation and energy expenditure. Physiol Behav. 2012;107:322–328. - PubMed
-
- Borbély AA, Achermann P. Sleep homeostasis and models of sleep regulation. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th. Elsevier Saunders; Philadelphia: 2005. pp. 405–417.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

