Crystal structure of the DNA cytosine deaminase APOBEC3F: the catalytically active and HIV-1 Vif-binding domain
- PMID: 23685212
- PMCID: PMC3805256
- DOI: 10.1016/j.str.2013.04.010
Crystal structure of the DNA cytosine deaminase APOBEC3F: the catalytically active and HIV-1 Vif-binding domain
Abstract
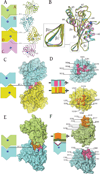
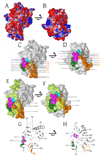
Human APOBEC3F is an antiretroviral single-strand DNA cytosine deaminase, susceptible to degradation by the HIV-1 protein Vif. In this study the crystal structure of the HIV Vif binding, catalytically active, C-terminal domain of APOBEC3F (A3F-CTD) was determined. The A3F-CTD shares structural motifs with portions of APOBEC3G-CTD, APOBEC3C, and APOBEC2. Residues identified to be critical for Vif-dependent degradation of APOBEC3F all fit within a predominantly negatively charged contiguous region on the surface of A3F-CTD. Specific sequence motifs, previously shown to play a role in Vif susceptibility and virion encapsidation, are conserved across APOBEC3s and between APOBEC3s and HIV-1 Vif. In this structure these motifs pack against each other at intermolecular interfaces, providing potential insights both into APOBEC3 oligomerization and Vif interactions.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

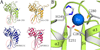

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

