MT1-MMP-dependent control of skeletal stem cell commitment via a β1-integrin/YAP/TAZ signaling axis
- PMID: 23685250
- PMCID: PMC3736823
- DOI: 10.1016/j.devcel.2013.04.011
MT1-MMP-dependent control of skeletal stem cell commitment via a β1-integrin/YAP/TAZ signaling axis
Abstract
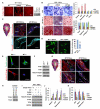
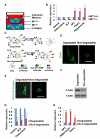
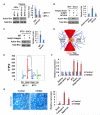
In vitro, topographical and biophysical cues arising from the extracellular matrix (ECM) direct skeletal stem cell (SSC) commitment and differentiation. However, the mechanisms by which the SSC-ECM interface is regulated and the outcome of such interactions on stem cell fate in vivo remain unknown. Here we demonstrate that conditional deletion of the membrane-anchored metalloproteinase MT1-MMP (Mmp14) in mesenchymal progenitors, but not in committed osteoblasts, redirects SSC fate decisions from osteogenesis to adipo- and chondrogenesis. By effecting ECM remodeling, MT1-MMP regulates stem cell shape, thereby activating a β1-integrin/RhoGTPase signaling cascade and triggering the nuclear localization of the transcriptional coactivators YAP and TAZ, which serve to control SSC lineage commitment. These data identify a critical MT1-MMP/integrin/YAP/TAZ axis operative in the stem cell niche that oversees SSC fate determination.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures




Comment in
-
Proteolytic remodeling of the ECM and the geometric control of stem cell fate.Dev Cell. 2013 May 28;25(4):325-6. doi: 10.1016/j.devcel.2013.05.012. Dev Cell. 2013. PMID: 23725758
Similar articles
-
Type I collagen deposition via osteoinduction ameliorates YAP/TAZ activity in 3D floating culture clumps of mesenchymal stem cell/extracellular matrix complexes.Stem Cell Res Ther. 2018 Dec 7;9(1):342. doi: 10.1186/s13287-018-1085-9. Stem Cell Res Ther. 2018. PMID: 30526677 Free PMC article.
-
Proteolytic remodeling of the ECM and the geometric control of stem cell fate.Dev Cell. 2013 May 28;25(4):325-6. doi: 10.1016/j.devcel.2013.05.012. Dev Cell. 2013. PMID: 23725758
-
β1 integrin-mediated signaling regulates MT1-MMP phosphorylation to promote tumor cell invasion.J Cell Sci. 2020 May 14;133(9):jcs239152. doi: 10.1242/jcs.239152. J Cell Sci. 2020. PMID: 32205364
-
[Regulation of differentiation of mesenchymal stem cells by the Hippo pathway effectors TAZ/YAP].Yi Chuan. 2013 Nov;35(11):1283-90. doi: 10.3724/sp.j.1005.2013.01283. Yi Chuan. 2013. PMID: 24579311 Review. Chinese.
-
The role of lamin A/C in mesenchymal stem cell differentiation.J Physiol Biochem. 2019 Feb;75(1):11-18. doi: 10.1007/s13105-019-00661-z. Epub 2019 Jan 31. J Physiol Biochem. 2019. PMID: 30706289 Review.
Cited by
-
Mechanics-driven nuclear localization of YAP can be reversed by N-cadherin ligation in mesenchymal stem cells.Nat Commun. 2021 Oct 28;12(1):6229. doi: 10.1038/s41467-021-26454-x. Nat Commun. 2021. PMID: 34711824 Free PMC article.
-
ADAMTS9-Regulated Pericellular Matrix Dynamics Governs Focal Adhesion-Dependent Smooth Muscle Differentiation.Cell Rep. 2018 Apr 10;23(2):485-498. doi: 10.1016/j.celrep.2018.03.034. Cell Rep. 2018. PMID: 29642006 Free PMC article.
-
A Review: Molecular Aberrations within Hippo Signaling in Bone and Soft-Tissue Sarcomas.Front Oncol. 2015 Sep 2;5:190. doi: 10.3389/fonc.2015.00190. eCollection 2015. Front Oncol. 2015. PMID: 26389076 Free PMC article. Review.
-
Integrated functions of membrane-type 1 matrix metalloproteinase in regulating cancer malignancy: Beyond a proteinase.Cancer Sci. 2017 Jun;108(6):1095-1100. doi: 10.1111/cas.13231. Epub 2017 May 22. Cancer Sci. 2017. PMID: 28267240 Free PMC article. Review.
-
Sphere-forming cells from peripheral cornea demonstrate the ability to repopulate the ocular surface.Stem Cell Res Ther. 2016 Jun 1;7(1):81. doi: 10.1186/s13287-016-0339-7. Stem Cell Res Ther. 2016. PMID: 27250558 Free PMC article.
References
-
- Anjos-Afonso F, Bonnet D. Isolation, culture, and differentiation potential of mouse marrow stromal cells. Curr. Protoc. Stem Cell. Biol. 2008;7:2B.3.1–2B.3.11. - PubMed
-
- Chan KM, Wong HL, Jin G, Liu B, Cao R, Cao Y, Lehti K, Tryggvason K, Zhou Z. MT1-MMP inactivates ADAM9 to regulate FGFR2 signaling and calvarial osteogenesis. Dev. Cell. 2012;22:1176–1190. - PubMed
-
- Chun TH, Hotary KB, Sabeh F, Saltiel AR, Allen ED, Weiss SJ. A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell. 2006;125:577–591. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

