HOT1 is a mammalian direct telomere repeat-binding protein contributing to telomerase recruitment
- PMID: 23685356
- PMCID: PMC3680732
- DOI: 10.1038/emboj.2013.105
HOT1 is a mammalian direct telomere repeat-binding protein contributing to telomerase recruitment
Abstract
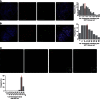
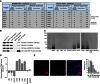
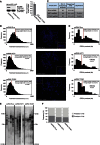
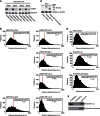
Telomeres are repetitive DNA structures that, together with the shelterin and the CST complex, protect the ends of chromosomes. Telomere shortening is mitigated in stem and cancer cells through the de novo addition of telomeric repeats by telomerase. Telomere elongation requires the delivery of the telomerase complex to telomeres through a not yet fully understood mechanism. Factors promoting telomerase-telomere interaction are expected to directly bind telomeres and physically interact with the telomerase complex. In search for such a factor we carried out a SILAC-based DNA-protein interaction screen and identified HMBOX1, hereafter referred to as homeobox telomere-binding protein 1 (HOT1). HOT1 directly and specifically binds double-stranded telomere repeats, with the in vivo association correlating with binding to actively processed telomeres. Depletion and overexpression experiments classify HOT1 as a positive regulator of telomere length. Furthermore, immunoprecipitation and cell fractionation analyses show that HOT1 associates with the active telomerase complex and promotes chromatin association of telomerase. Collectively, these findings suggest that HOT1 supports telomerase-dependent telomere elongation.
Conflict of interest statement
DK, F Butter, MM and F Buchholz declare a patent application of this work as patent PCT/EP2011/065943. The remaining authors declare that they have no conflict of interest.
Figures



Comment in
-
It's getting HOT at telomeres.EMBO J. 2013 Jun 12;32(12):1655-7. doi: 10.1038/emboj.2013.119. Epub 2013 May 17. EMBO J. 2013. PMID: 23685360 Free PMC article.
References
-
- Afonine PV, Grosse-Kunstleve RW, Adams PD (2005) The Phenix refinement framework. CCP4 Newslett 42:
-
- Araki M, Araki K, Yamamura K-I (2009) International Gene Trap Project: towards gene-driven saturation mutagenesis in mice. Curr Pharm Biotechnol 10: 221–229 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

