The importance of mixed selectivity in complex cognitive tasks
- PMID: 23685452
- PMCID: PMC4412347
- DOI: 10.1038/nature12160
The importance of mixed selectivity in complex cognitive tasks
Abstract
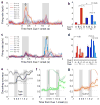
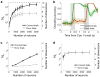
Single-neuron activity in the prefrontal cortex (PFC) is tuned to mixtures of multiple task-related aspects. Such mixed selectivity is highly heterogeneous, seemingly disordered and therefore difficult to interpret. We analysed the neural activity recorded in monkeys during an object sequence memory task to identify a role of mixed selectivity in subserving the cognitive functions ascribed to the PFC. We show that mixed selectivity neurons encode distributed information about all task-relevant aspects. Each aspect can be decoded from the population of neurons even when single-cell selectivity to that aspect is eliminated. Moreover, mixed selectivity offers a significant computational advantage over specialized responses in terms of the repertoire of input-output functions implementable by readout neurons. This advantage originates from the highly diverse nonlinear selectivity to mixtures of task-relevant variables, a signature of high-dimensional neural representations. Crucially, this dimensionality is predictive of animal behaviour as it collapses in error trials. Our findings recommend a shift of focus for future studies from neurons that have easily interpretable response tuning to the widely observed, but rarely analysed, mixed selectivity neurons.
Figures


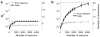
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

