A functional and perceptual signature of the second visual area in primates
- PMID: 23685719
- PMCID: PMC3710454
- DOI: 10.1038/nn.3402
A functional and perceptual signature of the second visual area in primates
Abstract
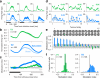
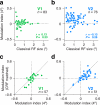
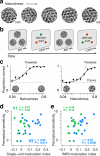
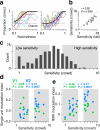
There is no generally accepted account of the function of the second visual cortical area (V2), partly because no simple response properties robustly distinguish V2 neurons from those in primary visual cortex (V1). We constructed synthetic stimuli replicating the higher-order statistical dependencies found in natural texture images and used them to stimulate macaque V1 and V2 neurons. Most V2 cells responded more vigorously to these textures than to control stimuli lacking naturalistic structure; V1 cells did not. Functional magnetic resonance imaging (fMRI) measurements in humans revealed differences between V1 and V2 that paralleled the neuronal measurements. The ability of human observers to detect naturalistic structure in different types of texture was well predicted by the strength of neuronal and fMRI responses in V2 but not in V1. Together, these results reveal a particular functional role for V2 in the representation of natural image structure.
Figures



References
-
- Schiller PH, Malpeli JG. The effect of striate cortex cooling on area 18 cells in the monkey. Brain Research. 1977;126:366–369. - PubMed
-
- Sincich LC, Horton JC. The circuitry of V1 and V2: integration of color, form, and motion. Annual Review of Neuroscience. 2005;28:303–326. - PubMed
-
- Hegdé J, Essen DCV. A comparative study of shape representation in macaque visual areas v2 and v4. Cerebral Cortex. 2007;17:1100–1116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

