Olfactory cortical neurons read out a relative time code in the olfactory bulb
- PMID: 23685720
- PMCID: PMC3695490
- DOI: 10.1038/nn.3407
Olfactory cortical neurons read out a relative time code in the olfactory bulb
Abstract
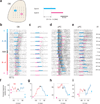
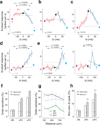
Odor stimulation evokes complex spatiotemporal activity in the olfactory bulb, suggesting that both the identity of activated neurons and the timing of their activity convey information about odors. However, whether and how downstream neurons decipher these temporal patterns remains unknown. We addressed this question by measuring the spiking activity of downstream neurons while optogenetically stimulating two foci in the olfactory bulb with varying relative timing in mice. We found that the overall spike rates of piriform cortex neurons (PCNs) were sensitive to the relative timing of activation. Posterior PCNs showed higher sensitivity to relative input times than neurons in the anterior piriform cortex. In contrast, olfactory bulb neurons rarely showed such sensitivity. Thus, the brain can transform a relative time code in the periphery into a firing rate-based representation in central brain areas, providing evidence for the relevance of a relative time-based code in the olfactory bulb.
Figures

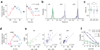




Comment in
-
Neural coding: timing is key in the olfactory system.Nat Rev Neurosci. 2013 Jul;14(7):458. doi: 10.1038/nrn3532. Epub 2013 Jun 5. Nat Rev Neurosci. 2013. PMID: 23736754 No abstract available.
Similar articles
-
The Interglomerular Circuit Potently Inhibits Olfactory Bulb Output Neurons by Both Direct and Indirect Pathways.J Neurosci. 2016 Sep 14;36(37):9604-17. doi: 10.1523/JNEUROSCI.1763-16.2016. J Neurosci. 2016. PMID: 27629712 Free PMC article.
-
Task-Demand-Dependent Neural Representation of Odor Information in the Olfactory Bulb and Posterior Piriform Cortex.J Neurosci. 2019 Dec 11;39(50):10002-10018. doi: 10.1523/JNEUROSCI.1234-19.2019. Epub 2019 Oct 31. J Neurosci. 2019. PMID: 31672791 Free PMC article.
-
Rapid Feedforward Inhibition and Asynchronous Excitation Regulate Granule Cell Activity in the Mammalian Main Olfactory Bulb.J Neurosci. 2015 Oct 21;35(42):14103-22. doi: 10.1523/JNEUROSCI.0746-15.2015. J Neurosci. 2015. PMID: 26490853 Free PMC article.
-
Odor representations in mammalian cortical circuits.Curr Opin Neurobiol. 2010 Jun;20(3):328-31. doi: 10.1016/j.conb.2010.02.004. Epub 2010 Mar 5. Curr Opin Neurobiol. 2010. PMID: 20207132 Free PMC article. Review.
-
Coding odor identity and odor value in awake rodents.Prog Brain Res. 2014;208:205-22. doi: 10.1016/B978-0-444-63350-7.00008-5. Prog Brain Res. 2014. PMID: 24767484 Free PMC article. Review.
Cited by
-
A Neuroscientific and Cognitive Literary Approach to the Treatment of Time in Calderón's Autos sacramentales.Front Integr Neurosci. 2022 Mar 28;16:780701. doi: 10.3389/fnint.2022.780701. eCollection 2022. Front Integr Neurosci. 2022. PMID: 35418840 Free PMC article.
-
Broadly tuned and respiration-independent inhibition in the olfactory bulb of awake mice.Nat Neurosci. 2014 Apr;17(4):569-76. doi: 10.1038/nn.3669. Epub 2014 Mar 2. Nat Neurosci. 2014. PMID: 24584050
-
High-Throughput Automatic Training System for Odor-Based Learned Behaviors in Head-Fixed Mice.Front Neural Circuits. 2018 Feb 13;12:15. doi: 10.3389/fncir.2018.00015. eCollection 2018. Front Neural Circuits. 2018. PMID: 29487506 Free PMC article.
-
Properties of an optogenetic model for olfactory stimulation.J Physiol. 2016 Jul 1;594(13):3501-16. doi: 10.1113/JP271853. Epub 2016 Mar 17. J Physiol. 2016. PMID: 26857095 Free PMC article.
-
Experience-dependent evolution of odor mixture representations in piriform cortex.PLoS Biol. 2023 Apr 25;21(4):e3002086. doi: 10.1371/journal.pbio.3002086. eCollection 2023 Apr. PLoS Biol. 2023. PMID: 37098044 Free PMC article.
References
-
- Wehr M, Laurent G. Odour encoding by temporal sequences of firing in oscillating neural assemblies. Nature. 1996;384:162–166. - PubMed
-
- VanRullen R, Guyonneau R, Thorpe SJ. Spike times make sense. Trends Neurosci. 2005;28:1–4. - PubMed
-
- Laurent G. A systems perspective on early olfactory coding. Science. 1999;286:723–728. - PubMed
-
- Hopfield JJ. Pattern recognition computation using action potential timing for stimulus representation. Nature. 1995;376:33–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

