Activity-dependent silencing reveals functionally distinct itch-generating sensory neurons
- PMID: 23685721
- PMCID: PMC3695070
- DOI: 10.1038/nn.3404
Activity-dependent silencing reveals functionally distinct itch-generating sensory neurons
Abstract
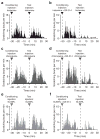
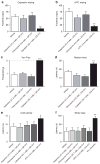
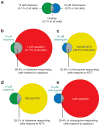
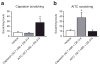
The peripheral terminals of primary sensory neurons detect histamine and non-histamine itch-provoking ligands through molecularly distinct transduction mechanisms. It remains unclear, however, whether these distinct pruritogens activate the same or different afferent fibers. Using a strategy of reversibly silencing specific subsets of murine pruritogen-sensitive sensory axons by targeted delivery of a charged sodium-channel blocker, we found that functional blockade of histamine itch did not affect the itch evoked by chloroquine or SLIGRL-NH2, and vice versa. Notably, blocking itch-generating fibers did not reduce pain-associated behavior. However, silencing TRPV1(+) or TRPA1(+) neurons allowed allyl isothiocyanate or capsaicin, respectively, to evoke itch, implying that certain peripheral afferents may normally indirectly inhibit algogens from eliciting itch. These findings support the presence of functionally distinct sets of itch-generating neurons and suggest that targeted silencing of activated sensory fibers may represent a clinically useful anti-pruritic therapeutic approach for histaminergic and non-histaminergic pruritus.
Conflict of interest statement
This research was funded, in part, by a research grant from Endo Pharmaceuticals, who have licensed the technology invented by authors B.P.B. and C.J.W.
Figures
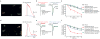
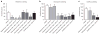
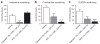
Comment in
-
Itching for relief.Nat Neurosci. 2013 Jul;16(7):775-7. doi: 10.1038/nn.3442. Nat Neurosci. 2013. PMID: 23799467 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

