Perivascular adipose tissue potentiates contraction of coronary vascular smooth muscle: influence of obesity
- PMID: 23685742
- PMCID: PMC3755741
- DOI: 10.1161/CIRCULATIONAHA.112.001238
Perivascular adipose tissue potentiates contraction of coronary vascular smooth muscle: influence of obesity
Abstract
Background: This investigation examined the mechanisms by which coronary perivascular adipose tissue (PVAT)-derived factors influence vasomotor tone and the PVAT proteome in lean versus obese swine.
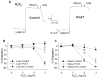
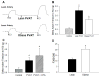
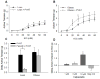
Methods and results: Coronary arteries from Ossabaw swine were isolated for isometric tension studies. We found that coronary (P=0.03) and mesenteric (P=0.04) but not subcutaneous adipose tissue augmented coronary contractions to KCl (20 mmol/L). Inhibition of CaV1.2 channels with nifedipine (0.1 µmol/L) or diltiazem (10 µmol/L) abolished this effect. Coronary PVAT increased baseline tension and potentiated constriction of isolated arteries to prostaglandin F2α in proportion to the amount of PVAT present (0.1-1.0 g). These effects were elevated in tissues obtained from obese swine and were observed in intact and endothelium denuded arteries. Coronary PVAT also diminished H2O2-mediated vasodilation in lean and, to a lesser extent, in obese arteries. These effects were associated with alterations in the obese coronary PVAT proteome (detected 186 alterations) and elevated voltage-dependent increases in intracellular [Ca(2+)] in obese smooth muscle cells. Further studies revealed that the Rho-kinase inhibitor fasudil (1 µmol/L) significantly blunted artery contractions to KCl and PVAT in lean but not obese swine. Calpastatin (10 μmol/L) also augmented contractions to levels similar to that observed in the presence of PVAT.
Conclusions: Vascular effects of PVAT vary according to anatomic location and are influenced by an obese phenotype. Augmented contractile effects of obese coronary PVAT are related to alterations in the PVAT proteome (eg, calpastatin), Rho-dependent signaling, and the functional contribution of K(+) and CaV1.2 channels to smooth muscle tone.
Keywords: adipose tissue; coronary disease; muscle, smooth; obesity; vasoconstriction.
Conflict of interest statement
Figures

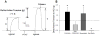


References
-
- Mahabadi AA, Reinsch N, Lehmann N, Altenbernd J, Kalsch H, Seibel RM, Erbel R, Mohlenkamp S. Association of pericoronary fat volume with atherosclerotic plaque burden in the underlying coronary artery: a segment analysis. Atherosclerosis. 2010;211:195–9. - PubMed
-
- Iacobellis G, Willens HJ. Echocardiographic epicardial fat: a review of research and clinical applications. J Am Soc Echocardiogr. 2009;22:1311–9. - PubMed
-
- Greif M, Becker A, von ZF, Lebherz C, Lehrke M, Broedl UC, Tittus J, Parhofer K, Becker C, Reiser M, Knez A, Leber AW. Pericardial adipose tissue determined by dual source CT is a risk factor for coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:781–6. - PubMed
-
- Boydens C, Maenhaut N, Pauwels B, Decaluwe K, Van d V. Adipose tissue as regulator of vascular tone. Curr Hypertens Rep. 2012;14:270–8. - PubMed
-
- Tesauro M, Cardillo C. Obesity, blood vessels and metabolic syndrome. Acta Physiol (Oxf) 2011;203:279–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

